INTRODUCTION
Pneumocystis carinii from rats are known to be genetically complexed. Pulsed-field gradient gel electrophoresis revealed two different stable karyotypes of P. carinii from rats which represented genetic diversity of this protists (Hong et al., 1990; Cushion et al., 1993). Pneumocystis carinii also showed antigenic variations between isolates found in humans and rats. Such variation is thought to be caused by different primary amino acid sequences and by different glycosylation mechanisms (Lundgrend, 1994). Furthermore, individual isolates of human P. carinii were also antigenically different (Kovacs et al., 1989). Serum antibody response to human P. carinii was also found to vary according to different geographical regions around the world. The variation was distinctive in the high molecular antigenic bands (Smulian et al., 1993). The different patterns of antibody reaction probably represent exposures to antigenically different strains of P. carinii. There are no data available regarding the antigenicity to human P. carinii in Korea; however, human serum , including the major antigenic determinant, IgG antibody, was shown to react with the antigen of rat P. carinii (Hong, 1991; Moon et al., 1995). The rat P. carinii in Korea showed two molecular karyotypes which represented the presence of genetic variation in the organism population (Hong et al., 1992). In addition to the different karyotypes, the antigenicity of rat P. carinii is expected to vary in Korea. To outline the biological characteristics of P. carinii in Korea, the present study investigated the antigenic diversity of rat derived P. carinii from four localities in Korea and compared the antigenicity to P. carinii in different localities with the use of ELISA and immunoblotting.
MATERIALS AND METHODS
Induction of P. carinii pneumonitis
Subject rats (Sprague-Dawley or Wistar strain, regardless of sex and age) were immuno-incompetently rendered with the injection of methylprednisolone acetate (DepoMedrol®, The Upjohn Co., Korea) 4 mg/week for 6 to 8 weeks. Drinking water was supplemented with ampicillin (1 mg/ml) or tetracycline (1 mg/ml) to suppress secondary bacterial infections. The rats were maintained at four localities from different vendors, Chunchon, Chungju, Kwangju, and Seoul during 1995-1996.
Isolation of rat-derived P. carinii and preparation of the antigen
Immunosuppressed rats were sacrificed, and the lungs were removed and examined by impression smears to determine the severity of infection. When there was no bacterial or fungal infection, and the density of P. carinii was enough (more than 10 cystic forms per field of ×1,000), the lungs were frozen at -70℃ in 10% glycerol phosphate buffered saline (pH 7.2) and were sent to the Department of Parasitology, Seoul National University College of Medicine, Seoul. The lungs were homogenized in a Stomacher blender (Seward Medical, UK) and purified as previously described (Hong, 1991; Moon et al., 1995). The purified sample was examined microscopically to determine the number and its purity, followed by homogenization for 30 sec using a sonicator (Ultrasonics W385, Farming Daily, NY, USA). Supernatant of the homogenate was used after 12,000 rpm centrifugation for 90 min at 4℃. This antigen was stored at -70℃ until used.
Collection of human sera
Human sera were also collected from four localities in 1995-1996. The sera from Chunchon were collected from a general population while those from Chungju and Kwangju were obtained from local hospitals. The sera collected from Seoul were from healthy students at a local medical school. The collected sera were stored at -70℃ until used.
Enzyme-Linked Immunosorbent Assay (ELISA)
The antigen was diluted to 2.5 µg/ml in carbonate buffer (pH 9.6), serum was diluted to 1:50 in PBS, and peroxidase conjugated anti-human IgG goat serum (Cappel Co., Durham, USA) was diluted to 1:5,000 in PBS. The substrate for the color reaction was OPD (o-phenylenediamine) at 4 mg/10 ml and the reaction was stopped with 50 µl 4 M H2SO4. The absorbance was recorded at 490 nm with an ELISA reader (Bio-Rad, Hercules, USA).
SDS-PAGE and immunoblot
The crude antigens were subjected to electrophoresis on 0.1% SDS and 7.5-15% gradient polyacrylamide gels. Twenty micrograms of the crude antigen were loaded to each well. Visible bands were then transferred onto a nitrocellulose membrane (Amersham, Buckinghamshire, UK) in a semi-blotter (Hoeffer, San Francisco, USA), followed by incubation with 1:50 diluted sera. Visualization of antigen-antibody reaction was obtained by adding peroxidase-conjugated anti-human IgG antibodies diluted to 1:5,000 (Jackson, West Glove, USA). The chromogenic substrate used was 4-chloro-1-naphthol (Sigma, St. Louis, USA) and the reaction was stopped by membrane washes with distilled water. Samples with O.D. values greater than 0.2 were subjected to immunoblotting.
RESULTS
ELISA with human sera
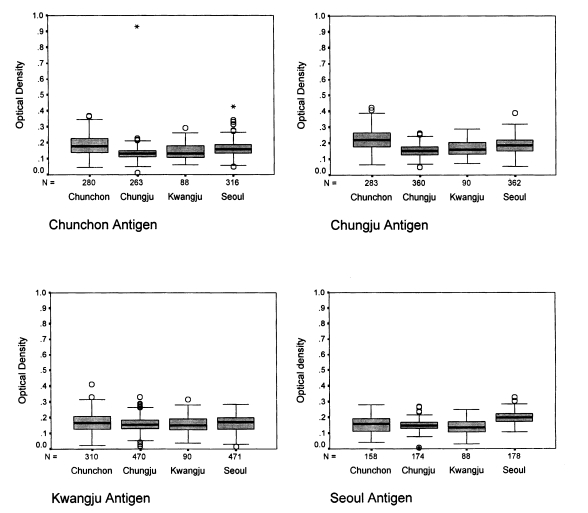
The absorbances of 1,294 human sera ranged from 0.02 to 0.36 (Fig. 1). When the Chunchon antigen was used, the absorbances were between 0.049 and 0.426 with sera from Chunchon, between 0.054 and 0.388 with sera from Chungju, between 0.016 and 0.268 with sera from Kwangju, and between 0.107 and 0.328 with sera from Seoul. The absorbances were significantly different in four localities (P<0.001), except for Chunchon and Kwangju (P=0.18). The absorbances were from 0.010 to 0.930 with sera from Chunchon, from 0.050 to 0.260 with sera from Chungju, from 0.020 to 0.332 with sera from Kwangju, and from 0.006 to 0.269 with sera from Seoul when Chungju antigen was used. The absorbances were different significantly by four localities (P<0.001) except between Seoul and Chungju (P=0.903), and between Chungju and Kwangju (P=0.191). When Kwangju antigen was applied, the absorbances were 0.063-0.292 with sera in Chunchon, 0.072-0.288 in Chungju, 0.038-0.316 in Kwangju, and 0.032-0.249 in Seoul. The absorbances were significantly different by four localities (P<0.001) except among Seoul, Chunchon, and Kwangju (P=0.06), and between Kwangju and Chungju (P=0.95). If Seoul antigen was subjected, the absorbances were 0.049-0.426 with sera in Chunchon, 0.054-0.388 in Chungju, 0.016-0.268 in Kwangju, and 0.107-0.328 in Seoul. The absorbances were significantly different by four localities (P<0.001) except between Chunchon and Kwangju (P=0.980).
Immunoblot reaction with human sera
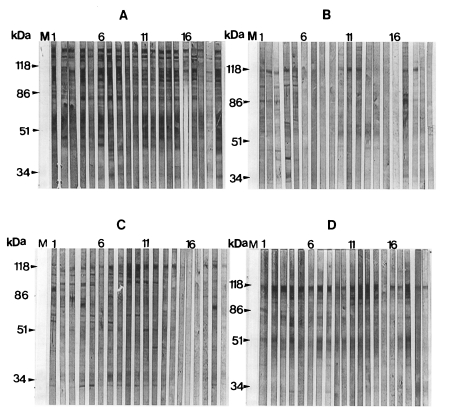
The typical patterns for immunoblotting between rat P. carinii antigens and human IgG antibodies are presented in Fig. 2, and their frequency values are summarized in Table 1. The reaction pattern to the 100 kDa band showed a significant difference among four localities when the Chunchon antigen was used (P=0.002). Other two bands revealed no significant difference (P=0.099 for 116 kDa, P=0.592 for 45-55 kDa bands). When the Chungju antigen was used, there was no significant difference in the reaction patterns to the three major bands found in four localities (P=0.367 for 116 kDa, P=0.181 for 100 kDa, P=0.583 for 45-55 kDa bands). When the Kwangju antigen was applied, there was also no significant difference (P=0.309 for 116 kDa, P=0.194 for 100 kDa, P=0.342 for 45-55 kDa bands). When we used the Seoul antigen, the reaction patterns to the 100 kDa band marked a significant difference among four localities (P=0.013). Other two bands showed no significance (P=0.878 for 116 kDa, P=0.104 for 45-55 kDa bands).
Of the three major antigenic bands found with the Seoul antigen, the 116 kDa band was most prominent while the 100 kDa band was weak and 45-55 kDa bands were diffuse, but their presence and staining intensity varied by the blots. The 116 kDa band was found most frequently from all localities, and the 100 kDa and 45-55 kDa bands occured at much lower frequency.
DISCUSSION
The major antigenic bands of human P. carinii include 30-40, 66, 95, 120, and more than 120 kDa bands. The serological reaction to these bands shows different patterns with respect to the different regions around the world (Smulian et al., 1993). Human and rat P. carinii are known to share common antigenic determinants, and the antigen of rat P. carinii in Korea showed cross-reactions to human serum in 116, 66, and 45-50 kDa bands (Moon et al., 1995). The cross-reaction is important for the serological application of rat P. carinii antigen. The present study showed three cross-reacting antigenic bands; 116, 100, and 45-55 kDa bands. The heterogeneous broad-based 45-55 kDa bands in the present study may be the same band as 45-50 kDa bands found by Moon et al. (1995). The 116 kDa band corresponds to the 110 or 120 kDa antigenic bands which have been found in human-, rabbit-, ferret-, and mouse-derived P. carinii (Linke et al., 1989; Bauer et al., 1993). It was known that IgG antibodies in rat sera react to 40-45, 50-55, 116, and 200 kDa bands of rat P. carinii (Hong et al., 1995).
The ELISA results showed that most absorbances were distributed close to 0.2 which indicated a normal distribution. The positive outcome of ELISA result is not so meaningful clinically, since all the population are usually exposed to P. carinii till two-year-old age (Hong, 1991). From the above results, it is shown that the absorbance of Chunchon sera was higher than the sera from other localities. This phenomenon may be due to the different exposure rate to the antigens in different localities. The test subjects in Chunchon lived in rural areas. They would be more susceptible to airborne rat P. carinii since the number of rats are higher in the rural area. An O.D. value of the Seoul sera to the Seoul antigen was higher than those from other localities, whereas it was consistently lower when the Seoul sera was used against the antigens from other localities. Such occurrence could be due to the fact that subjects who were medical students in Seoul had a higher chance of being exposed to P. carinii, since they experimented with laboratory rats. This phenomenon suggested that the immune reaction was stronger to the antigens from the same localities, which the antigens were exposed to the environment. As for the antigenic variation, the standard sera should be tested for several antigens. This kind of result can be also plotted from Fig. 1. However, the analysis is also same to that of Fig. 1. When the sera were fixed, there were significant differences shown as in Fig. 1. (data not shown).
Immunoblot analysis with sera from 130 normal children and 15 newborns in Korea revealed 40.0% of positive rate in specific IgG antibody reaction to the 40-55 and 116 kDa protein bands of P. carinii (Moon et al., 1995). In the present study, a high percentage of human sera from four localities reacted to the 116 kDa band. The reaction to the 100 and 45-55 kDa bands also occurred but less frequently. The frequencies of the reaction varied by the localities. The frequency variation of human serum reaction was mostly originated from the variation of 116 and 100 kDa bands of P. carinii antigen. It was known that the frequency of antibody reaction to antigens of 95, 120 and more than 120 kDa demonstrated significant geographical variation (Smulian et al., 1993). The present findings of ELISA and immunoblotting also showed that P. carinii carried different antigenic determinants by localities. It was already confirmed that P. carinii from Korean rats was a complex of genetically different organisms (Hong et al., 1992). It is more plausible that the locality variation may be due to the genetic difference. Even in the same locality, P. carinii of different antigenicity can co-exist. Human isolates of P. carinii also were known to be genetically diverse (Wakefield et al., 1994). Further studies are necessary to evaluate the antigenic variation with the genetic differences.
In conclusion, it is found that the reaction rate of the human sera to rat P. carinii antigen were different according to the localities. Also, the 116 kDa protein of rat P. carinii was the most frequent antigenic band reacting to human sera, though there were some variations by localities. This band is thought to be a primary target for immunological study of P. carinii from Korean rats.