INTRODUCTION
House dust mites (HDMs) have been well established as the most significant indoor sensitizing agents that are important in the induction of asthma and allergic rhinitis. In Korea, Ree et al. (1997) identified 23 species of mites from house dust mites of which Dermatophagoides farinae, D. pteronyssinus, and Tyrophagus putrescentiae, a storage mite, were reported to be the three most predominant species. However, it is generally believed that the predominant species are only D. farinae and D. pteronyssinus. Recently, several research groups have described the characterization of the allergens from two species of mites, especially the group 1 and 2 allergens. These two allergens are the most significant causative agents for the sensi-tization, invoking IgE and IgG antibody responses in 80% to 95% of patients allergic to HDMs, and each has been cloned and sequenced (Chapman and Platts-Mills, 1980; Krilis et al., 1984; Platts-Mills et al., 1992; Hakkaart et al., 1998a).
The crude allergen extracts were prepared from cultured mites, and they probably contained a complex mixture of proteins of which only a few was allergenic. Due to the lack of available methods in measuring allergenic components in the extracts, the potency of these extracts might vary from batch to batch (Platts-Mills and Chapman, 1991). One way to characterize allergen extracts is to measure their major allergen levels with monoclonal antibodies. Allergen-specific monoclonal antibodies have many advantages for allergen characterization, identification, and quantitation (Chapman, 1988; Ovsyannikova et al., 1994), since they possess a unique specificity and a high level of selectivity for a single epitope and can be produced in unlimited amount in vitro.
In this study, we describe the production of monoclonal antibodies and the analysis of Der p 2 epitopes. Consequently, this approach using monoclonal antibodies allowed a two-site capture ELISA to be developed.
MATERIALS AND METHODS
Recombinant Der p 2
A recombinant Der p 2 (rDer p 2) was obtained as previously described (Kim et al., 1999). Briefly, the full-length Der p 2 cDNA, of which the nucleotide sequences had been reported by Chua et al. (1990), was amplified by RT-PCR. Using a pGEX-4T3 expression vector (Pharmacia, Uppsala, Sweden), rDer p 2 was produced as a glutathione S-transferase (GST)-fusion protein. The fusion protein was purified by using a glutathione-agarose column (Pharmacia), and rDer p 2 was obtained by cutting GST off from a fusion protein using thrombin. About 1.2 mg of purified rDer p 2 was obtained from 1 L of bacterial culture.
Production of monoclonal antibodies
Monoclonal antibodies were generated as described previously (Yong et al., 1991). Briefly, SP 2/0 plasmacytoma cells were fused with spleen cells of BALB/c mice immunized with rDer p 2 mixed with an incomplete Freund's adjuvant three times at two weeks intervals. Hybridomas producing antibodies against rDer p 2 were identified by ELISA. Selected hybridomas were cloned by limiting dilution. Positive clones were expanded, and the supernatant was obtained.
Specificity and isotyping
Specificity of monoclonal antibodies were examined by ELISA using the crude extracts of D. farinae, a storage mite (T. putrescentiae), German cockroach, and a non-biting midge (Chironomus kiiensis).
Isotyping was carried out by ELISA with an isotyping kit for monoclonal antibodies according to the manufacturer's instruction (Sigma, St. Louis, USA).
Immunoblot analysis
Immunoblot analysis was performed according to the procedures described by Tsang et al. (1983) followed by SDS-PAGE. The membrane strips were reacted with undiluted culture supernatant and then incubated with 1:2,000 diluted goat alkaline phosphatase conjugated anti-mouse IgG (Sigma). Color was developed with a substrate for alkaline phosphatase (66 µl of 50 mg/ml of nitrobluetetrazolium and 33 µl of 50 mg/ml of 3-bromo-4-chloro-5-indolyl-phosphate in 100 mM Tris-HCl, pH 9.5, 100 mM NaCl, and 5 mM MgCl2).
Epitope analysis
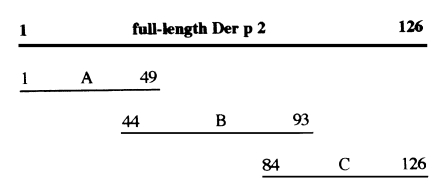
For epitope analysis, rDer p 2 comprising 126 amino acids (aa) was dissected into three fragments with several overlapping peptides, A (aa residues 1-49), B (44-93) and C fragment (84-126) (Fig. 1). In order to obtain these three dissected polypeptides, the three cDNA fragments were produced by PCR amplification and ligated into prokaryotic expression vectors. The cDNA for the A fragment was ligated into pGEX 4T-3 vector (Pharmacia), and cDNAs for the B and C fragments were ligated into pGEX 4T-1 vectors, respectively. Each recombinant polypeptide was expressed in E. coli and purified with a GST-affinity column. Immunoblot analysis was performed with each GST-fusion polypeptide against monoclonal antibodies.
HDM-specific IgE positive patients' sera were used to examine the reactivity of these polypeptides.
Purification of monoclonal antibodies
Two different monoclonal antibodies were purified by using a protein G affinity column (Pharmacia) according to the manufacturer's protocol. Briefly, culture supernatant equilibrated with 0.1 M phosphate buffered saline (PBS), pH 7.4, was passed through a column. The column was washed and bound protein was eluted with an elution buffer (0.5 M acetic acid, pH 3.0). Eluted fraction was neutralized by the addition of 2 M Tris, pH 9.0.
Labelling of horseradish peroxidase to a monoclonal antibody
Five mg of horseradish peroxidase (HRP) dissolved in 1.2 ml of water and 0.3 ml of 0.1 M sodium periodate in 10 mM sodium phosphate buffer, pH 7.0 was mixed. The mixture was incubated at the room temperature (RT) for 20 min and dialyzed overnight against 1 mM sodium acetate buffer (pH 4.0) at 4℃. Then, 0.5 ml of anti-Der p 2 monoclonal antibody (10 mg/ml) in 20 mM carbonate buffer, pH 9.5, was added to the solution and incubated at RT for 2 hr. After incubation, 100 µl of sodium borohydride in water (4 mg/ml) was added, reacted at 4℃ for 2 hr, and dialyzed in PBS overnight at 4℃. HRP conjugated monoclonal antibody was titrated to determine optimal dilutions in ELISA.
Measurement of Der p 2 by two-site capture ELISA
One of the purified anti-Der p 2 monoclonal antibody (5D10) was coated on a microtiter plate (10 µg/ml), using 0.1 M sodium bicarbonate buffer, pH 9.6, overnight at 4℃. After blocking the wells with 1% bovine serum albumin for 1 hr at 37℃, the rDer p 2 or HDM crude extracts with a serial dilution in PBS was incubated for 1 hr at 37℃. The plate was washed, and HRP-conjugated anti-Der p 2 monoclonal antibody (3B12) was added to the plate. After multiple washes, the wells were developed with 0.05% orthophenylenediamine and 0.006% hydrogen peroxide in 0.1 M phosphate-citrate buffer, pH 5.0. The color reaction was read at 490 nm with an ELISA reader.
RESULTS
Monoclonal antibodies to rDer p 2
Two-hundred seventy-four hybridomas were screened by ELISA. Thirteen clones (4.7%) were found to be positive. Finally, four clones, 3B12, 5D10, 4A5, and 2B6, which produced monoclonal antibodies with high affinity to rDer p 2, were established through a limiting dilution.
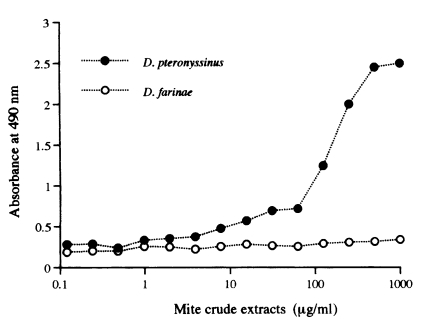
All four monoclonal antibodies were found to be highly species-specific. They reacted only with the rDer p 2 and D. pteronyssinus extracts, and did not cross-react with neither of any other arthropod extracts, especially D. farinae extract. Two of the monoclonal antibodies (3B12 and 2B6) were found to be IgG1 and the others (5D10 and 4A5) were IgM.
Epitope analysis
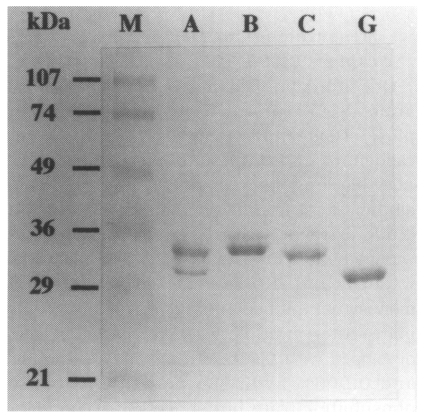
Recombinant polypeptides that were purified using a GST-affinity column are shown in Fig. 2.
As shown in Fig. 3, three monoclonal antibodies, 3B12, 2B6, and 4A5, showed reactivity to both B polypeptide and the full-length rDer p 2. Interestingly, however, monoclonal antibody 5D10 reacted only to the full-length rDer p 2. Reactivities were confirmed by immunoblot analysis (Fig. 4). Human sera also showed a reactivity to a B polypeptide and the full-length rDer p 2, but not with A or C polypeptide or GST, alone.
Two-site capture ELISA for Der p 2
Monoclonal antibodies used in this study were species-specific. Therefore, two-site capture ELISA could detect only Der p 2. The ELISA control curves were produced by the assay using rDer p 2 (Fig. 5). The limit of sensitivity of this assay was approximately 4 ng/ml with rDer p 2 (Fig. 5) and 8 µg/ml with the D. pteronyssinus crude extract (Fig. 6).
DISCUSSION
Sensitization to allergens produced by HDMs is commonly associated with symptoms of asthma and rhinitis around the world, and D. farinae and D. pteronyssinus are the major source of HDM allergens. Immunoblot analysis using patients' sera selected on the basis of high anti-mite IgE has revealed 32 different IgE-binding bands with molecular weights from 11 to over 100 kDa (Tovey and Baldo, 1987; Thomas and Smith, 1998). However, most recent researches have been focusing on the two major allergens (groups 1 and 2). The level of group 2 allergens in house dust mite has been reported to be as high as that of group 1 allergens (Yasueda et al., 1990). Although the group 1 allergens have been well known to be cysteine proteases (Chua et al., 1988), the group 2 allergens have not been clearly characterized as yet. In the past, there has been a speculation that the group 2 allergens are lysozymes, but it is now clear that they are not (Hakkaart et al., 1997). They are similar in amino acid sequences, size, and the distribution of cysteine residues to a family of epididymal proteins (Thomas and Smith, 1998). However, anti-group 2 antibodies have been reported to show reactivity associated with the gut (van Hage-Hamsten et al., 1995). More characterizations of the group 2 allergens are needed, especially on the functions and distributions in the mites.
In a previous study, we successfully produced rDer p 2 and found that rDer p 2 had a good solubility and high IgE-reactivity (Kim et al., 1999). This recombinant protein could be easily obtained from bacteria as pure form in a large quantity which makes it useful for the allergy skin test. In this study, monoclonal antibodies against rDer p 2 have been produced from BALB/c mice in an ordinary way, even though the poor antibody response of BALB/c mice to group 2 allergens has been reported previously (Heymann et al., 1989; Ovsyannikova et al., 1994). Nevertheless, it was not very difficult to produce monoclonal antibodies against rDer p 2 from the strain of mouse. Interestingly, the isotype of two monoclonal antibodies were IgM, although the reason of high incidence of IgM producing hybridomas was not clear. In this study, fragmented polypeptides as well as the full-length recombinant protein from bacteria were used for the epitope analysis of monoclonal antibodies. As shown in the result, each monoclonal antibody reacted to the identical or different regions of the molecule. Especially, the polypeptide comprising aa residues 44-93 of rDer p 2 was highly reactive with most monoclonal antibodies as well as human IgE.
Monoclonal antibody-based immunoassays have been used to measure allergen contents in the crude extracts of HDMs and have been applied for monitoring and assessing mite allergen contamination in indoor environment (Ovsyannikova et al., 1994). We produced monoclonal antibodies for similar purposes in this study. Initially, we aimed to produce monoclonal antibodies to cross-reactive epitopes of group 2 mite allergens. Not as expected, monoclonal antibodies produced were highly species-specific and did not show any cross-reactivities to Der f 2. While Der p 2 and Der f 2 have been reported to show almost complete cross-reactivity (Yasueda et al., 1989), some difference has been also noted (Lind et al., 1987). The cDNA sequences of Der f 2 and Der p 2 have been reported to show polymorphisms (Trudinger et al., 1991; Chua et al., 1996), and variant-specific monoclonal antibodies have been described as for Der f 2 (Hakkaart et al., 1998b). Therefore, high species-specificity of monoclonal antibodies produced in this study were not very unusual phenomena, and we could develop an assay using monoclonal antibodies specifically to measure Der p 2. In the near future, we would need to produce other monoclonal antibodies, which react specifically to Der f 2 or react equally well with both Der p 2 and Der f 2, in order to measure a total group 2 allergens. However, the sensitivity of ELISA developed in this study was comparable to the widely used assay that was reported previously (Ovsyannikova et al., 1994). The monoclonal antibodies against rDer p 2 will be used for the further characterization of Der p 2, and the ELISA developed in this study will be applied for environmental monitoring of indoor allergens and for the standardization of mite allergen extracts.