AbstractSustained-releasing praziquantel (SRP) tablet was designed for single dose treatment regimen of clonorchiasis. A previous pre-clinical study confirmed its sustained-releasing characteristics and a better cure rate than conventional praziquantel (PZQ). In this clinical study, the pharmacokinetics of this SRP tablet were investigated in human volunteers (phase 1; 12 volunteers), and its curative efficacy was examined in clonorchiasis patients (phase 2; 20 volunteers). In the phase 1 clinical study, blood concentrations of both tablets showed wide individual variation. The AUClast of SRP was 497.9 ± 519.0 ng · hr/ml (mean ± SD) and PZQ of 628.6 ± 695.5 ng · hr/ml, and the AUCinf of SRP was 776.0 ± 538.5 ng · hr/ml and of PZQ 658.6 ± 709.9 ng · hr/ml. Cmax values of SRP and PZQ were 90.7 ± 82.2 ng/ml and 214.9 ± 251.9 ng/ml, and Tmax values were 3.42 ± 1.43 hr and 1.96 ± 1.23 hr, respectively. SRP tablets showed similar AUC values, but lower Cmax and longer Tmax values than PZQ. In the phase 2 study, SRP at 30 mg/kg (single dose) achieved a 60% cure rate and a 95.5% egg reduction rate. The cure rate of a single dose SRP was unsatisfactory compared with that of the conventional PZQ dose, but much better than that achieved by a single dose PZQ.
INTRODUCTIONClonorchiasis is an infection caused by the Chinese liver fluke Clonorchis sinensis, and is one of the major human parasitic trematode infections in East Asia, i.e., China, Korea, Eastern Russia, Taiwan and Vietnam. Approximately 20 million people are estimated to be infected by C. sinensis worldwide, of whom over 15 million are Chinese (Lun et al., 2005) and more than one million are Korean (Korea Association of Health Promotion, 2004). Clonorchiasis induces several pathological changes in the infected bile ducts, such as, duct dilatation, mucosal hyperplasia, periductal fibrosis, purulent cholangitis and abscess formation, cholelithiasis, and cholangiocarcinoma (Hong, 2003). Clonorchiasis is a major health problem that requires resolution in endemic areas.
Praziquantel (PZQ) is a derivative of pyraziniso-quinolone, and is the drug of choice for treatment of trematode and cestode infections. However, although approximately 80% of oral PZQ is absorbed in the intestinal tract, only a small proportion reaches the systemic circulation in an unchanged form because of extensive first-pass metabolism (Andrews, 1985). It is commonly used to treat clonorchiasis or other fluke diseases for more than 20 years, and recommended to use 3 doses of 25 mg/kg (total 75 mg/kg) for treatment of clonorchiasis (Rim, 1986). However, this schedule is difficult to adhere to because of the 5 hr interval requirement, which is essential for cure. Furthermore, second and third doses are frequently forgotten, which resulted in incomplete cure (Hong et al., 2001). In order to overcome this shortcoming, development of an alternative/modified drug, such as sustained-releasing praziquantel (SRP) tablet is highly required (Hong et al., 2003).
The SRP tablet used in the present study, incorporates a high viscosity hydroxypropyl methylcellulose (HPMC) base, which extends tablet dissolution over 10 hr (Hong et al., 2003). Moreover, the tablet showed sustained-releasing characteristics and slow absorption with improved anthelmintic efficacy against C. sinensis as compared with conventional PZQ in experimental dogs. Therefore, the devised SRP tablet appeared a suitable candidate for a single dose preclinical study. During this prior work in a canine model, SRP tablets showed pharmacokinetic characteristics of prolonged Tmax (time to peak plasma concentration) and a reduced Cmax (peak plasma concentration), i.e., the half-life of praziquantel (t1/2) is substantially determined by its rate of gastrointestinal absorption.
In the present study, we undertook to confirm sustained-release of SRP in humans, and to define the single dose medication requirement for clonorchiasis in humans. Thus, we compared the pharmacokinetic characteristics and the safety aspects of PZQ and SRP in healthy volunteers by phase 1 study. In the following phase 2 study, 30 mg/kg of SRP was administered to clonorchiasis patients and cure rates and egg reduction rates were determined.
MATERIALS AND METHODSPhase 1 clinical trialThe phase 1 study was approved by the institutional review board of Seoul National University Hospital, Seoul, Korea. The pharmacokinetic characteristics of SRP tablets were analyzed in 14 healthy volunteers in the Clinical Trial Center, Seoul National University Hospital in January, 2001. The inclusion criteria for healthy volunteers were; 1) age range 20-40 years, 2) male volunteers relatively close (± 15%) to ideal body weight, 3) no abnormal medical history, physical examination, ECG or other laboratory findings, 4) able to complete the trial. All volunteers willingly participated in this trial. Six volunteers in each group completed the trial; two volunteers dropped because of side effects or contract violations.
Conventional PZQ tablets (Distocide®, Shin Poong Pharmaceutical Co. Ltd. Seoul, Korea) containing 600 mg of praziquantel and SRP tablets containing 300 mg praziquantel (supplied by Shin Poong Pharmaceutical Co.) were orally administered to healthy volunteers. The SRP formula was identical to that described previously (Hong et al., 2003). In group I (the reference group), PZQ 600 mg was administered orally, and this was followed by SRP 600 mg one week later. In contrast, volunteers in group II (the trial group) were administered orally with drugs in reverse order, i.e., SRP 600 mg, and then PZQ 600 mg one week later.
Pharmacokinetic parameters, such as, area under the plasma concentration-time curve from 0 hr to the final observation time (AUClast), area under the plasma concentration-time curve from 0 hr to infinity (AUCinf), Cmax, Tmax, t1/2, the volume of distribution (Vd/F), oral clearance (CL/F), and mean residence time (MRT) were analyzed using a non-compartment model. The safety of SRP was evaluated according to subjective and objective symptoms, a physical examination, and vital signs. Point and 90% confidence interval estimations were evaluated to compare the difference or ratio of SRP and PZQ, and pharmacokinetic data were analyzed statistically using SAS® 9.1 and Equivtest® 1.0. Carry-over effects, inter-subject variation within the group, period effects, and product effects were assayed using analysis of variance (ANOVA).
Phase 2 clinical trialInstitutional review board approved this clinical trial at the Inje University Pusan Paik Hospital, Busan, Korea, and 20 clonorchiasis patients were recruited for the phase 2 study at Inje University Pusan Paik Hospital between October 2003 and December 2004. Prior to therapy information on the phase 2 study was provided to all patients, and all agreed to join the study. Patients were interviewed to determine medical histories and a physical examination was performed on each subject. Data were recorded on case report forms. Patients' feces were screened for C. sinensis eggs using the Kato-Katz method and only egg positive individuals were included (Hong et al., 2003). Patients were administered orally 30 mg/kg of SRP, and subjects were re-screened for eggs 30 days after administration. Chemotherapeutic effects were evaluated from cure and egg reduction rates.
RESULTSPhase 1 clinical trialFourteen volunteers received both test (SRP 600 mg) and reference drugs (PZQ 600 mg). Two were excluded because of a contract violation or side effects. The remaining 12 volunteers completed the phase 1 clinical trial (6 volunteers/group), and their pharmacokinetic data were subjected to per-protocol analysis.
No significant differences were observed between groups I and II in terms of demographic information, such as, age, weight, or height. Group I was composed of 6 men, mean age 24.4 years, mean body weight 70.2 kg, and mean height 177.0 cm, and the corresponding data for the 6 men in group II were 23.3 years, 70.7 kg, and 174.2 cm, respectively.
Four of the 14 volunteers experienced side effects after SRP or PZQ administration. One subject was excluded after PZQ administration due to flushing, abdominal discomfort, and soft stools. Three persons showed rhinorrhea, vomiting and palpitation after SRP treatment. However, these side effects were not significantly different in those administered SRP or PZQ in terms of severity and frequency, and all resolved spontaneously. However, both PZQ and SRP showed large inter-subject absorption variations.
The AUClast, AUCinf, Cmax, Tmax values of SRP and PZQ are shown in Table 1. The t1/2 values of SRP and PZQ were 4.56 ± 2.35 hr and 3.07 ± 2.76 hr (mean ± SD), respectively, and of MRT were 5.64 ± 1.77 hr and 3.03 ± 0.85 hr. SRP showed a significant increase in Tmax (P = 0.026) and MRT (P = 0.002) versus PZQ. Variance analysis of AUClast and Cmax showed no carry-over effect, period effect, and product effect. The area under the plasma concentration-time curve (AUC) for SRP was not significantly different from that of PZQ, but whilst SRP showed a similar absorption amount, it also had a lower Cmax, and a longer Tmax than PZQ, which had a slower drug absorption rate. The CL/F values were similar for the 2 drugs (CL/F ratio of SRP/PZQ = 1.16). In contrast, the Vd/F of SRP was greater than that of PZQ (Vd/F ratio = 1.96). However, no carry-over effect, period effect, and product effect was observed in the 2 pharmacokinetic parameters of both drugs.
When administered as single equivalent dose (1,800 mg of SRP or PZQ), SRP was found to produce a higher serum concentration 6 hr after administration (Fig. 1). As shown by the 90 percentile drug concentration range, serum concentration variations were high due to both high inter-subject variations and the remaining drug concentrations.
Phase 2 clinical trialThe phase 2 study involved 20 clonorchiasis patients. Their demographic characteristics were as follows; median age 72.5 years (range, 35-75 years), 72.5% males, average eggs per gram of feces (EPG) before treatment 3,612.4 (range, 42-16,527). A single dose of 30 mg/kg SRP produced a 60% cure rate and a 95.5% egg reduction with an average EPG of 163.8. Five patients developed side effects; 3 dizziness only and 2 dizziness and nausea. However, these symptoms subsided spontaneously.
DISCUSSIONPraziquantel is not always effective in treating C. sinensis infection due to poor compliance of patients although its anthelmintic efficacy is known to be excellent, which is attributed to the need for 3 divided doses at 5 hr intervals (Hong et al., 2001). There is an obvious need for a single dose regimen for successful control of clonorchiasis in endemic areas. The SRP tablets produced using praziquantel and HPMC showed retarded PZQ release characteristics; nevertheless, SRP tablets were found to consistently and completely dissolve over 10 hr in artificial intestinal solution (Hong et al., 2003). This finding was confirmed by sustained-release of PZQ and a low rate of absorption in the human intestine during the phase 1 clinical trial of the present study, which also confirms our earlier observations in dogs (Hong et al., 2003). Moreover, both SRP and PZQ are safe when administered alternatively in the phase 1 study, and in particular, no neurological side effects were observed.
Considering the pharmacokinetic results of the phase 1 study, the AUCinf of SRP was larger than that of PZQ, but the AUClast of PZQ was larger than that of SRP. Moreover, the AUClast/AUCinf ratios of SRP and PZQ were 86% and 92%, respectively, which suggests that the observation period was sufficient for both. On the other hand, AUC values were similar for the 2 drugs. These AUCinf and AUClast results are explained by the longer terminal half-life of SRP than conventional PZQ, because the terminal half-life is determined by the absorption rate, and the SRP tablet is more slowly released and absorbed, and thus has a longer terminal half-life. Therefore, the two forms were absorbed at similar extents, according to the AUC data.
Pharmacokinetic analysis performed during the phase 1 trial revealed that Cmax was lower for SRP, although this was not significant, whereas Tmax was significantly prolonged for SRP. These characteristics indicate a reduction in SRP concentration fluctuation, and suggest that SRP could be useful as a single dosage control regimen in clonorchiasis. In our previous study in beagle dogs, the Cmax of SRP was 10.3 µg/0.25 ml, but that of PZQ was 2.5 µg/0.25 ml, which was significantly different (Hong et al., 2003). In the present study, however, Cmax of SRP was lower than that of PZQ (P = 0.084). These contrary findings might be attributed to absorption and metabolism differences in humans and dogs, which need to be investigated further.
Side effects of PZQ depend on its peak serum concentrations (Lee, 1984; Rim, 1986). According to previous studies, single dosage of conventional PZQ should be administered at less than 1,800 mg. However, when SRP tablets are administered, peak serum praziquantel concentrations are reduced versus conventional PZQ tablets. Therefore the SRP tablet allows the single dosage to be increased, and hence, a revised dosage of SRP was utilized in the present phase 2 clinical study.
The oral administration of three consecutive doses of 25 mg/kg PZQ at 5-hr-interval in a day (the recommended standard regimen), achieves clonorchiasis cure rates of 83-85% (Rim et al., 1981; Seo et al., 1983). In Korea a single dose of 40 mg/kg PZQ had been applied for mass clonorchiasis control, and this achieved a 25.0% cure rate and an egg reduction rate of 95.5% (Lee, 1984). However, in clonorchiasis control trials, actual cure rate is not as high as expected due to poor compliance with the three dose regimen (Hong et al., 1998, 2001). In our phase 2 clinical trial, the cure rate achieved using a single dose of 30 mg/kg SRP was unsatisfactory. Although our single dose SRP medication showed a cure rate of 60% and 95.5% egg reduction rate, it presents a convenient and effective means for mass treatment. In this context, a single dose of 30 mg/kg SRP might be preferred for mass clonorchiasis control from the viewpoints of cure and egg reduction rates.
The present SRP tablet appears to be a promising candidate for the control of bovine schistosomiasis in endemic areas. Buffalos are reservoirs of Schistosoma japonicum, and therefore, control at this level is essentially required for the disease control. Moreover, the buffalo bowel is long enough to ensure dissolution of the present SRP tablet, and therefore, SRP tablets may be able to achieve improved cure rates of bovine schistosomiasis by a single dose administration.
The release kinetics of SRP in vitro suggests that gastrointestinal transit time may have influenced cure rates for clonorchiasis. The average gastrointestinal transit time is about 5 hr in humans, and ranges from 1.5 hr to 10 hr, when analyzed by capsule endoscopy (Velayos Jimenez et al., 2005). Thus, when a person with gastrointestinal transit time of 5 hr takes a SRP tablet, half of the PZQ in the SRP tablet may not be absorbed in the small intestine, but pass out of the body unabsorbed. Moreover, the amount of drug absorbed in the colon may be negligible. Therefore, cure and egg reduction rates may depend on individual gastrointestinal transit times. The maintenance of praziquantel concentrations in blood at over a certain threshold for 10 hr is important for cure of clonorchiasis rather than AUC. Therefore, a new design of one-dose tablet that can maintain a stable praziquantel concentration is needed. In addition, a tablet with a faster release rate than SRP is required, to ensure compatibility with the average gastrointestinal transit time of 5 hr in man. This can probably be achieved by modifying the HPMC concentration (Fu et al., 2004). In addition, other biodegradable polymers should be considered as matrix materials for new SRP tablets, e.g., poly DL-lactide (PLA) and poly DL-lactide-co-glycolide (PLGA).
In conclusion, the present SRP tablet has good sustained-releasing characteristics, and single dose SRP at 30 mg/kg may offer the possibility of improved cure and egg reduction rates in clonorchiasis. The SRP tablet looks promising to bovine schistosomiasis, which should be studied further.
ACKNOWLEDGMENTSThis study was supported financially by the Shin Poong Pharmaceutical Co., Seoul, Korea, which also supplied SRP tablets with permission from the Korea Food and Drug Administration.
REFERENCES1. Andrews P. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol Ther. 1985. 29:129-156. PMID: 3914644.
2. Fu XC, Wang GP, Liang WQ, Chow MS. Prediction of drug release from HPMC matrices: effect of physicochemical properties of drug and polymer concentration. J Control Release. 2004. 95:209-216. PMID: 14980769.
3. Hong ST. In Miliotis MD, Bier JW eds, Clonorchis sinensis. International Handbook of Foodborne Pathogens. 2003. New York, USA: Marcell Dekker, Inc.; 581-592.
4. Hong ST, Choi MH, Kim CH, Chung BS, Ji Z. The Kato-Katz method is reliable for diagnosis of Clonorchis sinensis infection. Diagn Microbiol Infect Dis. 2003. 47:345-347. PMID: 12967748.
5. Hong ST, Lee SH, Lee SJ, Kho WG, Lee M, Li S, Chung BS, Seo M, Choi MH. Sustained-release praziquantel tablet: pharmacokinetics and the treatment of clonorchiasis in beagle dogs. Parasitol Res. 2003. 91:316-320. PMID: 14574562.
6. Hong ST, Rim HJ, Min DY, Li X, Xu J, Feng Z, Lee SH. Control of clonorchiasis by repeated treatments with praziquantel. Korean J Parasitol. 2001. 39:285-292. PMID: 11775328.
7. Hong ST, Yoon K, Lee M, Seo M, Choi MH, Sim JS, Choi BI, Yun CK, Lee SH. Control of clonorchiasis by repeated praziquantel treatment and low diagnostic efficacy of sonography. Korean J Parasitol. 1998. 36:249-254. PMID: 9868890.
8. Korea Association of Health Promotion. Prevalence of intestinal parasitic infections in Korea - The Seventh Report. 2004. Seoul, Korea.
9. Lee SH. Large scale treatment of Clonorchis sinensis infection with praziquantel under field conditions. Arzneim-Forsch/Drug Res. 1984. 34:1227-1230. PMID: 6542401.
10. Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005. 5:31-41. PMID: 15620559.
11. Rim HJ. The current pathobiology and chemotherapy of clonorchiasis. Korean J Parasitol. 1986. 24(suppl):1-141.
12. Rim HJ, Lyu KS, Lee JS, Joo KH. Clinical evaluation of the therapeutic efficacy of praziquantel (Embay 8440) against Clonorchis sinensis infection in man. Ann Trop Med Parasitol. 1981. 75:27-33. PMID: 7023402.
13. Seo BS, Lee SH, Chai JY, Hong ST. Praziquantel (Distocide@) in treatment of Clonorchis sinensis infection. Korean J Parasitol. 1983. 21:241-245.
14. Velayos Jimenez B, Fernandez Salazar L, Aller de la Fuente R, de la Calle Valverde F, Del Olmo Martinez L, Arranz Santos T, Gonzalez Hernandez J. Study of gastronitestinal transit times with capsule endoscopy. Gastroenterol Hepatol. 2005. 28:315-320. PMID: 15989811.
Fig. 1Mean (standard deviation) plasma concentrations after the oral administration of a single dose (600 mg tablet) of sustained-releasing (SR) formulation (SRP, closed circle) and after the similar administration of conventionally formulated praziquantel (open circle) in 14 subjects. 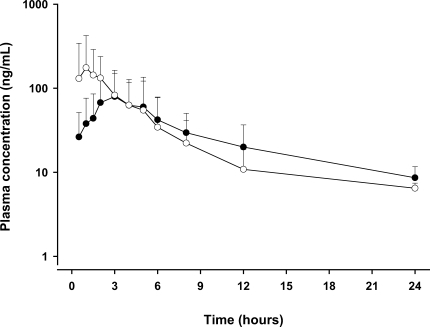
Table 1.Summary of pharmacokinetic parametersa) after an oral administration of 600 mg praziquantel (PP analysis)
a) AUClast, area under the plasma concentration-time curve from 0 hr to final observation time; AUCinf, area under the plasma concentration-time curve from 0 hr to infinity; Cmax, peak plasma concentration; Tmax, time to peak plasma concentration. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||