AbstractA 45-year-old-male who had underlying ulcerative colitis and presented with fever and dry cough. Initially, the patient was considered to have invasive aspergillosis due to a positive galactomannan assay. He was treated with amphotericin B followed by voriconazole. Nevertheless, the patient deteriorated clinically and radiographically. The lung biopsy revealed eosinophilic pneumonia, and ELISA for Toxocara antigen was positive, leading to a diagnosis of pulmonary toxocariasis. After a 10-day treatment course with albendazole and adjunctive steroids, the patient recovered completely without any sequelae. Pulmonary toxocariasis may be considered in patients with subacute or chronic pneumonia unresponsive to antibiotic agents, particularly in cases with eosinophilia.
INTRODUCTIONHuman toxocariasis is a zoonosis caused by Toxocara canis or by Toxocara catis larvae [1]. Humans are infected through ingestion of embryonated eggs from contaminated soil, raw vegetables, raw meat, or pet hair [2,3]. Toxocara migrans in the lungs is usually asymptomatic or accompanied by mild, non-specific symptoms associated with transient pulmonary infiltration [4,5]. Here, we describe a severe case of pulmonary toxocariasis mimicking invasive aspergillosis in an adult who had been treated with immunosuppressants for ulcerative colitis.
CASE REPORTA 45-year-old male presented with fever, cough, and non-purulent sputum, which had persisted over a 3-month period. The patient had ulcerative colitis that had been well controlled with azathioprine and mesalazine for the previous 5 years. Prior to hospitalization, he had been treated with antibiotics at a local clinic for 2 weeks without clinical improvement.
On physical examination, the patient appeared chronically ill with a heart rate of 72 beats/min, respiratory rate of 20 breaths/min, blood pressure of 130/75 mmHg, and body temperature of 38.2℃. Lung auscultation revealed crackles in the right lung field. Initial laboratory tests showed a normal leukocyte count and mild anemia (Table 1). Serum C-reactive protein levels (CRP, 109.8 mg/L) and erythrocyte sedimentation rate (ESR, 116 mm/h) were elevated, but the increase in procalcitonin (0.085 ng/ml) was not remarkable. Arterial blood gas analysis and liver function tests were within normal limits.
Chest X-ray and computed tomography (CT) showed large consolidations in the right upper lobe, middle lobe, and lower lobe superior segments (Fig. 1A, 2A). Bronchoalveolar lavage (BAL) was performed through the right upper and lower superior segmental bronchi. Microbiological investigation of the sputum and BAL fluid for bacteria, mycobacteria (Ziehl-Neelsen stain, culture, and PCR), and fungi were all negative. Only Aspergillus serum antigen (galactomannan assay) was positive with a value of 0.76 (optical density cutoff value, ≥0.5). With a diagnosis of probable invasive aspergillosis, considering the patient's immune status and positive galactomannan assay, amphotericin B (1.0 mg/kg/day) was administered for 8 days. However, the patient's symptoms worsened, and the chest CT findings showed deterioration (Fig. 2B).
After percutaneous lung biopsy, voriconazole (4 mg/kg, intravenous every 12 hr) was given to the patient on the 9th day of hospitalization due to concerns regarding resistance to amphotericin B. However, despite voriconazole treatment, the patient complained of dry cough with persistent fever, and the size of the right-side pleural effusion increased (Fig. 1B). A pleural effusion assay showed eosinophil-dominant exudates with a protein of 4,600 mg/dl, lactate dehydrogenase (LDH) of 643 U/L, adenosine deaminase (ADA) of 13.6 U/L, and a whole blood cell (WBC) count of 1,400/µl (eosinophils 40%, neutrophils 25%, and lymphocytes 35%). At that time, peripheral complete blood cell (CBC) count also revealed eosinophilia, which had not been detected initially (Table 1). Stool smears for parasitic analyses were negative, but Toxocara serology was strongly positive (IgG antibody optical density, 2.059; cut off value, ≥0.879) in ELISA. Lung pathology showed acute and chronic granulomatous inflammation with many eosinophils (Fig. 3).
The patient was diagnosed with pulmonary toxocariasis. After a 10-day treatment course with albendazole (400 mg b.i.d.) and prednisolone (0.5 mg/kg/day), the patient's symptoms improved rapidly and follow-up chest X-ray revealed complete resolution (Fig. 1C). The patient recovered without any sequelae.
DISCUSSIONThe clinical spectrum of human toxocariasis is diverse, encompassing asymptomatic infection, ocular migrans, neurologic migrans, and visceral migrans (hepatic and pulmonary infection) [2,4,6]. Owing to non-specific manifestations, the diagnosis of toxocariasis is frequently missed or delayed. The seroprevalence of toxocariasis is high in the general population. It is reported to be 5% in Korean adults and varies according to geographic area: 2.4% in the USA, 4.0% in Switzerland, 20.5% in Brazil, 21.6% in Iran, and 30.4% in Nigeria [7,8,9]. Ingestion of raw meat and uncooked cow liver is still popular in Korea. In this case, the patient had ingested raw beef meat, but did not have close contact with pets. The prevalence and clinical significance of toxocariasis needs to be further investigated.
In adult Korean patients with toxocariasis, lung involvement was reported in 22.7% [10]. On lung histology, eosinophil-dominant inflammatory responses are observed during the early stage, while histiocytes are collected around the degenerating larvae, occasionally forming granulomas in the late stages [11]. Eosinophilic lung disease is a typical manifestation of pulmonary toxocariasis; however, this condition can be induced by a heterogeneous group of disorders, including infections (especially parasitic and fungal), adverse drug reactions, connective tissue diseases, allergic bronchopulmonary aspergillosis, Churg-Strauss syndrome, and paraneoplastic syndrome from lymphoma or metastatic disease [12,13]. Although histopathologic findings and peripheral eosinophilia may be useful in diagnosing toxocariasis, Toxocara larvae are rarely identified in the tissue. Thus, serological tests (ELISA for Toxocara antigen) are required for the diagnosis of toxocariasis.
In this study, a false positive galactomannan assay might have contributed to the delayed diagnosis of pulmonary toxocariasis. A false-positive galactomannan assay can be caused by antibiotics (most commonly piperacillin-tazobactam, amoxicillin or amoxicillin-clavulanate) or intravenous fluids containing sodium gluconate [14,15]. False positive results may occur in patients infected with fungi containing a cross-reactive galactomannan (e.g., Histoplasma capsulatam) [16]. In addition, numerous foods (pasta, rice, soybean protein, etc.) contain galactomannan, so damage to the gut wall may enable translocation of galactomannan from the gut lumen into blood, thereby producing false-positive results [17,18]. In the present case, the patient had neither concurrent infection by cross-reactive microorganisms nor had he received aforementioned antibiotic agents. However, the patient had been suffering from ulcerative colitis, so the false-positive results may have arisen from an influx of galactomannan-degraded products through the disrupted intestinal mucosal barrier.
As previously reported, most cases of pulmonary toxocariasis are asymptomatic, manifesting as transient and migratory pulmonary infiltrates [19]. In symptomatic cases, a short course of albendazole treatment (400 mg twice daily for 5 days) is recommended, and adjunctive steroid therapy produces rapid improvement in the clinical course [2]. However, recent clinical studies showed a low cure rate (32%) and recurrence after 5-day albendazole therapy [20,21]. For this reason, some reports recommend treating toxocariasis with albendazole for 4 weeks or longer [22].
In summary, pulmonary toxocariasis should be considered in patients with subacute or chronic pneumonia unresponsive to antibiotic agents, particularly in cases with eosinophilia. Further studies are required to establish the optimal duration of albendazole therapy, which may need to be stratified based on the transmission routes, infection site, and disease severity.
REFERENCES1. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 2003;16:265-272. PMID: 12692098.
2. Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol 2001;39:1-11. PMID: 11301585.
3. Yoshikawa M, Koyama N, Hontsu S, Yamamoto Y, Mikasa K, Kimura H. Lessons from eight cases of adult pulmonary toxocariasis: abridged republication. Respirology 2011;16:1014-1015. PMID: 21609365.
4. Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010;104:3-23. PMID: 20149289.
5. Yoon YS, Lee CH, Kang YA, Kwon SY, Yoon HI, Lee JH, Lee CT. Impact of toxocariasis in patients with unexplained patchy pulmonary infiltrate in Korea. J Korean Med Sci 2009;24:40-45. PMID: 19270811.
6. Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol 2001;75:299-305. PMID: 11818044.
7. Abdi J, Darabi M, Sayehmiri K. Epidemiological situation of toxocariasis in Iran: meta-analysis and systematic review. Pak J Biol Sci 2012;15:1052-1055. PMID: 24261119.
8. Fan CK, Hung CC, Du WY, Liao CW, Su KE. Seroepidemiology of Toxocara canis infection among mountain aboriginal schoolchildren living in contaminated districts in eastern Taiwan. Trop Med Int Health 2004;9:1312-1318. PMID: 15598263.
9. Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol 2002;40:113-117. PMID: 12325440.
10. Kim YJ, Kyung SY, An CH, Lim YH, Park JW, Jeong SH, Lee SP, Choi DC, Jeong YB, Kang SY. The characteristics of eosinophilc lung diseases cause by Toxocara canis larval infestation. Tuberc Respir Dis 2007;62:19-26.
11. Inoue K, Inoue Y, Arai T, Nawa Y, Kashiwa Y, Yamamoto S, Sakatani M. Chronic eosinophilic pneumonia due to visceral larva migrans. Intern Med 2002;41:478-482. PMID: 12135183.
12. Katz U, Shoenfeld Y. Pulmonary eosinophilia. Clin Rev Allergy Immunol 2008;34:367-371. PMID: 18197481.
13. Wechsler ME. Pulmonary eosinophilic syndromes. Immunol Allergy Clin North Am 2007;27:477-492. PMID: 17868860.
14. Aubry A, Porcher R, Bottero J, Touratier S, Leblanc T, Brethon B, Rousselot P, Raffoux E, Menotti J, Derouin F, Ribaud P, Sulahian A. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J Clin Microbiol 2006;44:389-394. PMID: 16455889.
15. Surmont I, Stockman W. Gluconate-containing intravenous solutions: another cause of false-positive galactomannan assay reactivity. J Clin Microbiol 2007;45:1373. PMID: 17287325.
16. Wheat LJ, Hackett E, Durkin M, Connolly P, Petraitiene R, Walsh TJ, Knox K, Hage C. Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin Vaccine Immunol 2007;14:638-640. PMID: 17344352.
17. Murashige N, Kami M, Kishi Y, Fujisaki G, Tanosaki R. False-positive results of Aspergillus enzyme-linked immunosorbent assays for a patient with gastrointestinal graft-versus-host disease taking a nutrient containing soybean protein. Clin Infect Dis 2005;40:333-334. PMID: 15655766.
18. Ansorg R, van den Boom R, Rath PM. Detection of Aspergillus galactomannan antigen in foods and antibiotics. Mycoses 1997;40:353-357. PMID: 9470421.
19. Kang YR, Kim SA, Jeon K, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ, Kang ES, Um SW. Toxocariasis as a cause of new pulmonary infiltrates. Int J Tuberc Lung Dis 2013;17:412-417. PMID: 23407232.
20. Sturchler D, Schubarth P, Gualzata M, Gottstein B, Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol 1989;83:473-478. PMID: 2694978.
21. Kim MH, Jung JW, Kwon JW, Kim TW, Kim SH, Cho SH, Min KU, Kim YY, Chang YS. A case of recurrent toxocariasis presenting with urticaria. Allergy Asthma Immunol Res 2010;2:267-270. PMID: 20885912.
22. Yoshikawa M. Duration of treatment with albendazole for hepatic toxocariasis. Nat Clin Pract Gastroenterol Hepatol 2009;6:E1-E2. PMID: 19259103.
Fig. 1Chest X-rays showing consolidations in the right upper lobe, middle lobe, and lower lobe superior segment at initial presentation (A), increased extent of consolidations with right-sided pleural effusion on the 10th day of hospitalization (B), and complete resolution after a 10-day treatment course with albendazole and adjunctive steroid (C). 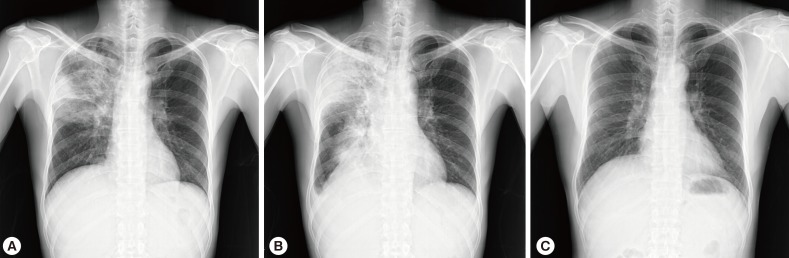
Fig. 2Chest computed tomography scans in the lung window setting showing large consolidations in the right upper lobe, middle lobe, and lower lobe superior segment at initial presentation (A) and increased extent on the 9th day after hospitalization (B). 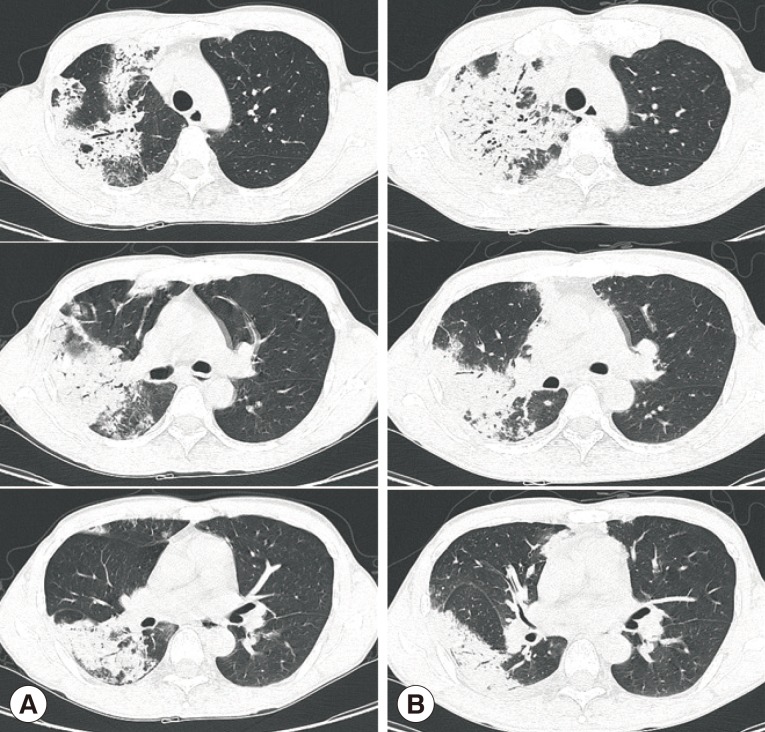
Fig. 3Lung pathology showing acute and chronic granulomatous inflammation with many eosinophils (H&E stain, A:×100, B:×400). 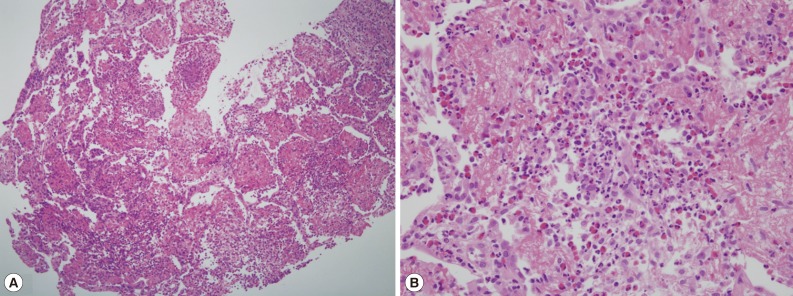
Table 1.Laboratory findings at initial presentation and during treatment |
|
|||||||||||||||||||||||||||||||||||||