AbstractToxoplasmosis, caused by Toxoplasma gondii, is a parasitic zoonosis with worldwide distribution. The present study investigated the prevalence of T. gondii in dogs in Zhanjiang city, southern China, using both serological and molecular detection. A total of 364 serum samples and 432 liver tissue samples were collected from the slaughter house between December 2012 and January 2013 and were examined for T. gondii IgG antibody by ELISA and T. gondii DNA by semi-nested PCR based on B1 gene, respectively. The overall seroprevalence of T. gondii IgG antibody was 51.9%, and T. gondii DNA was detected in 37 of 432 (8.6%) liver tissue samples. These positive DNA samples were analyzed by PCR-RFLP at 3'- and 5'-SAG2. Only 8 samples gave the PCR-RFLP data, and they were all classified as type I, which may suggest that the T. gondii isolates from dogs in Zhanjiang city may represent type I or type I variant. This study revealed the high prevalence of T. gondii infection in dogs in Zhanjiang city, southern China. Integrated measures should be taken to prevent and control toxoplasmosis in dogs in this area for public health concern.
Toxoplasma gondii is an obligate intracellular protozoan parasite that has a worldwide distribution and infects a wide range of warm-blooded vertebrates, including humans and dogs [1]. Domestic cats and other felids are the only known definitive hosts of this parasite since they can excrete oocysts into the environment. According to recent statistical data, T. gondii prevalence has been found in one third of the world population [2]. Humans get infected with T. gondii through ingesting undercooked meat containing tissue cysts or water or food contaminated with T. gondii oocysts, or by occasionally ingesting oocysts from the environment [3]. The dog, an intermediate host for T. gondii, is important in the epidemiology of this parasite because they can serve as sentinels of environmental contamination with oocysts and can be used to demonstrate the infection pressure to other hosts, including humans [4,5].
T. gondii isolates in North America and Europe present a highly clonal population structure mainly consisted of 4 lineages, namely type I, II, III, and 12 [6-9], which have genetic and biological differences from that in South America [10-12]. Several studies have documented the genotype of T. gondii isolates from different animals in China [13-15], but information about genotyping of T. gondii isolates from dogs in China is limited. Therefore, the current study was conducted to determine the prevalence of T. gondii infection in dogs from Zhanjiang, southern China, and to study the genotypes of the T. gondii isolates.
A total of 364 blood samples and 432 liver tissue samples were collected from dogs in Zhanjiang city, southern China, between December 2012 and January 2013. Dogs were randomly selected from the dog farms. These dogs are farmed for meat and are mainly consumed by the local people. One blood sample or 1 liver tissue sample was collected from a dog. However, the blood samples did not correspond to the tissue samples by the number due to the constraint of field condition. When the dog was slaughtered, tissue samples were collected, and blood samples were drawn from jugular vein into a sterile, plain centrifuge tube. Then, the tissue samples were saved in the microtubes at 4˚C. The blood samples were left to clot at room temperature for 6 hr and centrifuged at 3,000 rpm for 10 min. The separated sera were stored at -20˚C until needed for ELISA. This study was approved by the Animal Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (permit code, LVRIAEC2012-007). Dogs were handled in strict accordance with the Animal Ethics Procedures and Guidelines of the People's Republic of China.
IgG antibodies to T. gondii were determined using a commercially available ELISA kit (Haitai, Zhuhai, Guangdong Province, China) according to the manufacturer’s recommendations. A serum sample was considered positive when the value was 1.1 times higher than the mean value of positive control, negative control, and blank control. Genomic DNA was extracted from these liver tissues using TIANamp Genomic DNA kit (TianGen™, Beijing, China) according to manufacturer’s recommendations and previous descriptions [15]. Then, a semi-nested PCR based on B1 gene was employed to detect the T. gondii DNA following the previously described method [16]. B1 gene positive DNA samples were submitted to nested PCR amplification of 3'- and 5'- SAG2 [17,18], followed by digestion with restriction enzymes MboI and Hha1, respectively. The products were resolved in 2.5-3.0% agarose gel to display restriction fragment length polymorphisms (RFLP) using a gel document system (UVP GelDoc-It™ Imaging System, Cambridge, UK). Six T. gondii strains, namely GT, PTG, CTG, MAS, TgCatCa1, and TgCatBr5, were used as references.
Of the 364 serum samples of dogs in Zhanjiang city, southern China, 189 (51.9%) reacted positively. A previous study reported a seroprevalence of 70.9% in 175 stray dogs housed in shelters at Umuarama city, Brazil [19]. Alvarado-Esquivel et al. [20] reported a high seroprevalence of 67.3% in dogs in Veracruz, Mexico. The overall seroprevalence (51.9%) of T. gondii in dogs in Zhanjiang city was lower than that of Umuarama and Veracruz, but much higher than that observed in other parts of China, such as Guangzhou [21], Lanzhou [22], Kunming [23], and Shenyang [24]. High prevalence of T. gondii infection in dogs in Zhanjiang city reported here indicates a high environmental contamination with oocysts. Cats are the only definitive hosts of T. gondii, playing a significant role in the transmission of this parasite [1]. During the present investigation, we observed that cats were present on the farm and had free access to the dogs and their feed. This may represent a major risk factor for T. gondii infection in the dog farm.
T. gondii DNA was detected in 37 of the 432 (8.6%) liver tissue samples by using semi-nested PCR targeting the B1 gene. The target fragment was about 130 bp in length (Fig. 1). Four of these positive PCR products were randomly selected and sent to sequencing. Sequence comparison and analysis revealed 100% homology with the published T. gondii B1 gene sequence (GenBank accession no. AF179871). Genotyping of positive DNA samples was performed by employing PCRRFLP technique. Due to low DNA concentration, only 8 of 37 positive DNA samples gave the PCR-RFLP data on 3'- and 5'-SAG2, and they were identified as type I (Fig. 2). The results of genotyping of these isolates and 6 references are summarized in Table 1.
ELISA is among the most commonly used methods for investigation of IgG antibody. IgG antibodies usually appear within 1-2 weeks of acquisition with T. gondii infection, peak within 1-2 months, decline at various rates, and usually persist for life. Because of its high sensitivity and specificity, low cost, and ease of practice, ELISA is widely used for diagnosis of T. gondii infection. In the present study, seroprevalence of IgG antibodies (51.9%) does not keep in agreement with the prevalence of T. gondii DNA (8.6%). Actually, IgG antibody based seroprevalence mainly reflects that exposure of dogs to T. gondii infection in the investigated geographic area may be very common, while DNA detection reveals the presence of viable T. gondii in dogs and that they may be mostly acute infections. New detection methods such as IgG antibody avidity test and more studies are needed to explore the T. gondii infection details in dogs in Zhanjiang city. Anyway, what we could confirm in this study was that the dog farm is seriously contaminated with T. gondii oocysts. Urgent measures should be taken to prevent infection from spreading.
Limited data about genotyping of T. gondii isolates from dogs in China is available. A previous study reported the genotype of T. gondii isolates from dogs in Henan province and considered it as a type I variant [25]. The present result shared the same type at the (3'+5') SAG2 loci with T. gondii isolates from dogs in Henan [25]. This may suggest that T. gondii isolates from dogs in Zhanjiang city may belong to type I or a type I variant. However, further studies of sampling more dog samples from wider geographical locations are needed to draw a valid conclusion.
The present survey showed that T. gondii prevalence in dogs in Zhanjiang city, southern China is high. The dog meat is consumed in this region by the local people, and T. gondii is considered as an important food-borne parasite. Thus, dogs can serve as a transport host for T. gondii to humans. Therefore, it is essential to implement integrated measures to prevent and control T. gondii infection in dogs. Moreover, it is urgent to improve the eating habit of the local people and implement T. gondii-inspection during dog slaughtering and processing.
ACKNOWLEDGEMENTSThe project support was provided in part by the National Natural Science Foundation of China (grant no. 31228022) and the Science Fund for Creative Research Groups of Gansu Province (grant no. 1210RJIA006). Assoc. Prof. Chunlei Su at Department of Microbiology, the University of Tennessee, Knoxville, USA is thanked for providing reference Toxoplasma gondii DNA samples used in the present study.
REFERENCES1. Dubey JP. Toxoplasmosis of animals and humans. 2nd ed. Boca Raton, Florida, USA. CRC Press. 2010.
2. Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol 2009;39:895-901.
3. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2012;25:264-296.
4. Sedlak K, Bartova E. The prevalence of Toxoplasma gondii IgM and IgG antibodies in dogs and cats from the Czech Republic. Veterinarni Medicina 2006;12:555-558.
5. Lindsay DS, Dubey JP. Toxoplasma gondii-the model apicomplexan: perspectives and methods. London, UK. Academic Press. 2007, pp 133-152.
6. Darde ML, Bouteille B, Pestre-Alexandre M. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J Parasitol 1992;78:786-794.
7. Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 1995;172:1561-1566.
8. Ajzenberg D, Banuls AL, Tibayrenc M, Darde ML. Microsatellite analysis of Toxoplasma gondii shows considerable polymorphism structured into two main clonal groups. Int J Parasitol 2002;32:27-38.
9. Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, Sibley LD. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol 2011;41:645-655.
10. Dubey JP, Graham DH, Blackston CR, Lehmann T, Gennari SM, Ragozo AM, Nishi SM, Shen SK, Kwok OC, Hill DE, Thulliez P. Biological and genetic characterisation of Toxoplasma gondii isolates from chickens (Gallus domesticus) from Sao Paulo, Brazil: unexpected findings. Int J Parasitol 2002;32:99-105.
11. Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proc Natl Acad Sci USA 2006;103:11423-11428.
12. Dubey JP, Sundar N, Hill D, Velmurugan GV, Bandini LA, Kwok OC, Majumdar D, Su C. High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA. Int J Parasitol 2008;38:999-1006.
13. Dubey JP, Zhu XQ, Sundar N, Zhang H, Kwok OC, Su C. Genetic and biologic characterization of Toxoplasma gondii isolates of cats from China. Vet Parasitol 2007;145:352-356.
14. Cong W, Huang SY, Zhou DH, Zhang XX, Zhang NZ, Zhao Q, Zhu XQ. Prevalence and genetic characterization of Toxoplasma gondii in house sparrows (Passer mesticus) in Lanzhou, China. Korean J Parasitol 2013;51:363-367.
15. Jiang HH, Huang SY, Zhou DH, Zhang XX, Su C, Deng SZ, Zhu XQ. Genetic characterization of Toxoplasma gondii from pigs from different localities in China by PCR-RFLP. Parasit Vectors 2013;6:227.
16. Hill DE, Chirukandoth S, Dubey JP, Lunney JK, Gamble HR. Comparison of detection methods for Toxoplasma gondii in naturally and experimentally infected swine. Vet Parasitol 2006;141:9-17.
17. Honnré S, Couvelard A, Garin YJF, Bedel C, Hénin D, Derouin F. Génotypage de souches de Toxoplasma gondii chez des patients immunodéprimés. Pathol Biol 2000;48:541-547.
18. Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 2010;137:1-11.
19. de Paula Dreer MK, Gonçalves DD, da Silva Caetano IC, Gerônimo E, Menegas PH, Bergo D, Ruiz Lopes-Mori FM, Benitez A, de Freitas JC, Evers F, Navarro IT, Martins Lde A. Toxoplasmosis, leptospirosis and brucellosis in stray dogs housed at the shelter in Umuarama municipality, Paraná, Brazil. J Venom Anim Toxins Incl Trop Dis 2013;19:23.
20. Alvarado-Esquivel C, Romero-Salas D, Cruz-Romero A, GarcíaVázquez Z, Peniche-Cardeña A, Ibarra-Priego N, Ahuja-Aguirre C, Pérez-de-León AA, Dubey JP. High prevalence of Toxoplasma gondii antibodies in dogs in Veracruz, Mexico. BMC Vet Res 2014;10:191.
21. Zhang H, Zhou DH, Chen YZ, Lin RQ, Yuan ZG, Song HQ, Li SJ, Zhu XQ. Antibodies to Toxoplasma gondii in stray and household dogs in Guangzhou, China. J Parasitol 2010;96:671-672.
22. Wu SM, Huang SY, Fu BQ, Liu GY, Chen JX, Chen MX, Yuan ZG, Zhou DH, Weng YB, Zhu XQ, Ye DH. Seroprevalence of Toxoplasma gondii infection in pet dogs in Lanzhou, Northwest China. Parasit Vectors 2011;4:64.
23. Duan G, Tian YM, Li BF, Yang JF, Liu ZL, Yuan FZ, Zhu XQ, Zou FC. Seroprevalence of Toxoplasma gondii infection in pet dogs in Kunming, Southwest China. Parasit Vectors 2012;5:118.
Fig. 1.Representative PCR products of T. gondii B1 gene by semi-nested PCR from liver tissue DNA samples of dogs. M represents a DNA marker. Lane 1 represents positive control. Lanes 2-9 represent positive amplification. Lane 10 represents negative control. 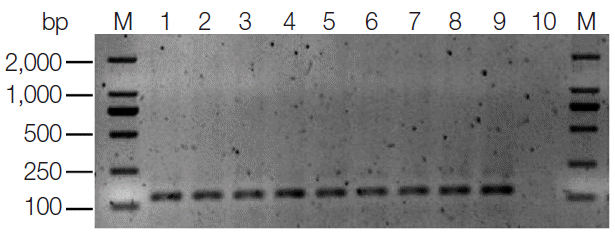
Fig. 2.PCR-RFLP analyses of T. gondii isolates from dogs in Zhanjiang, southern China based on 3'-SAG2 and 5'-SAG2. M represents a DNA marker. Lanes 1-14 represent GT1, PTG, CTG, MAS, TgCatCaI, TgCaBr5, TgDZJ1, TgDZJ2, TgDZJ3, TgDZJ4, TgDZJ5, TgDZJ6, TgDZJ7, and TgDZJ8, respectively. 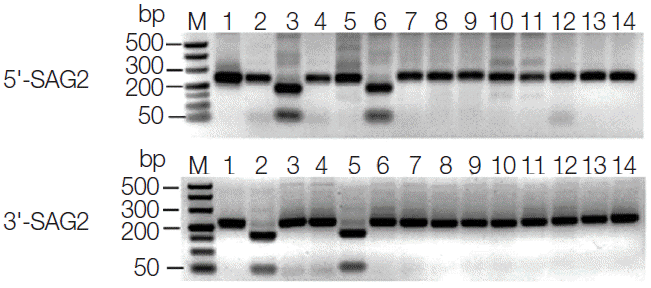
Table 1.Summary of genotyping of T. gondii isolates from dogs in Zhanjiang city, southern China |
|
|||||||||||||||||||||||||||||||||||||||||