AbstractIntegrated control strategies are important for sustainable control of schistosomiasis and soil-transmitted helminthiasis, despite their challenges for their effective implementation. With the support of Good Neighbors International in collaboration with National Institute of Medical Research, Mwanza, Tanzania, integrated control applying mass drug administration (MDA), health education using PHAST, and improved safe water supply has been implemented on Kome Island over 5 years for controlling schistosomiasis and soil-transmitted helminths (STHs). Baseline surveys for schistosomiasis and STHs was conducted before implementation of any integrated control strategies, followed by 4 cross-sectional follow-up surveys on randomly selected samples of schoolchildren and adults in 10 primary schools and 8 villages, respectively, on Kome islands. Those follow-up surveys were conducted for impact evaluation after introduction of control strategies interventions in the study area. Five rounds of MDA have been implemented from 2009 along with PHAST and improved water supply with pumped wells as other control strategies for complementing MDA. A remarkable steady decline of schistosomiasis and STHs was observed from 2009 to 2012 with significant trends in their prevalence decline, and thereafter infection rate has remained at a low sustainable control. By the third follow-up survey in 2012, Schistosoma mansoni infection prevalence was reduced by 90.5% and hookworm by 93.3% among schoolchildren while in adults the corresponding reduction was 83.2% and 56.9%, respectively. Integrated control strategies have successfully reduced S. mansoni and STH infection status to a lower level. This study further suggests that monitoring and evaluation is a crucial component of any large-scale STH and schistosomiasis intervention.
INTRODUCTIONSchistosomiasis (particularly due to Schistosoma mansoni infection) and soil-transmitted helminthiasis (STHs) are serious public health problems in the lake zone area of Lake Victoria and its associated islands as one of the most affected areas in Tanzania [1]. Despite this, no serious large scale control program has been implemented in the integrated manner and sustainable way; most previously attempted control programmes in Lake Victoria basin have either been in a small scale [2] or solely mass drug administration (MDA) with no other control strategies [3].
Schistosomiasis and STHs control programs typically have been mainly focusing on the provision of preventive chemotherapy (with praziquantel and albendazole) as advocated by WHO [4] and in Lake Victoria zone projects have mainly targeted on schoolchildren until recently [5]. Only in Ukerewe Islands, all community members had opportunity of being covered with MDA [3], while other equally highly affected islands (e.g., Kome Island) and other lake shore communities were left out. Unfortunately, this control programme that had just lasted only for 2 years comprised of MDA as the only control strategy. Although praziquantel (PZQ) is playing an important role in controlling schistosomiasis in much of sub-Saharan Africa [6], it is unlikely alone to have a lasting impact on transmission [6,7]; therefore, other complementary control strategies are needed. The short term impact of MDA does not prevent reinfection, which may occur rapidly post-treatment, nor does it likely to be sustainable in control of schistosomiasis and STHs [8]. Hence, integrated efforts for control of schistosomiasis and STHs that include safe water supply and health education for improving sanitation and preventive measures are most likely to be much more effective and sustainable [9,10]. Health education strategies have been proven effective in achieving behavior change [11-13]. Recently, there has been a dream of moving from morbidity control to elimination of schistosomiasis; in such circumstances integrated control has got great potential role to play.
With the support of Good Neighbors International (GNI) in collaboration with National Institute of Medical Research (NIMR), Mwanza Centre, Tanzania, an integrated control program of schistosomiasis and STHs in the Lake zone was established. The control strategies of schistosomiasis and STHs that have been employed for this integrated control programme in Kome Island since 2009 include: MDA annually to all community members (≥4 years old) using praziquantel (PZQ) and albendazole (ALB), construction of pumped wells for provision of alternative safe water supply since 2010, and provision of health education among adult community members using Participatory Hygiene and Sanitation Transformation (PHAST) approach since early 2011.
The parasitological and morbidity baseline survey of intestinal schistosomiasis and STHs was conducted before implementing the mentioned control strategies in February 2009. Monitoring of the impact of the first round MDA by cross-sectional survey was done in March 2010 (13 months post MDA intervention) followed by consecutive follow-up surveys almost annually up to the fifth one in July 2013 for monitoring of the other rounds of MDA and other control strategies (PHAST and improved water supply). This work reports a comparative trend of infection status during the baseline and consecutive 5 follow-up surveys post MDA along with PHAST and improved water over 5 years to assess the impact of integrated control strategies on intestinal schistosomiasis and hookworm infection status on Kome Island, Tanzania.
MATERIALS AND METHODSStudy area and populationKome Island is the 5th largest island (after Ukerewe, Rubondo, Ukara, and Mwaisome) in Lake Victoria out of 49% of the lake surface controlled by Tanzania. The island is in Sengerema District situated at longitudinal 32024” and 32024 east and latitude 2014’ and 2025”. According to the 2002 National census, the Kome Island had a population of 38,062 with average growth rate of 2.9%. Administratively, it has 2 wards, 9 villages, and 53 sub-villages. It is served by 1 health centre and 2 dispensaries. It has about 10 primary schools and 2 secondary schools in each ward, with almost each village having a primary school, with an exception of Lugata village which has 3 primary schools.
The source of water for drinking, washing, and other chores is Lake Victoria, natural wells, and streams. The lake harbors the snail intermediate hosts for Schistosoma, mainly Biomphalaria choanomphala and Biomphalaria sudanica [1]. The highest transmission areas of Lake Victoria are in the eastern (in Mara region) and southwestern part (in Mwanza and Geita regions) of Lake Victoria, particularly on the islands. Occupationally most of the people are engaged in peasant farming and fishing activities. They grow cassava, maize, and sweet potatoes as food crops and cotton as a cash crop. Livestock include cattle, goats, chickens, ducks, and a few pigs. All the occupational as well as recreational activities have obvious bearing to acquisition and transmission of intestinal schistosomiasis, STHs, and other parasitic diseases like malaria and amebiasis in the study area. Marked increase of commercial fisheries of Nile perch (Lates niloticus) fillets and ‘Dagaas’ (Rastrineobola argentous) from mid-1990’s would have resulted in increased water contact activities to the local fishermen and fish processors, aggravated further to the acquisition and transmission of schistosomiasis in the study areas.
Previous programs toward schistosomiasis and STHs in the study area was the way back in 1989 (by SAREC) which had just lasted for 5 years, and was mainly research-based with selected treatment for only positive cases for egg excretion. This was followed by the National Schistosomiasis and STHs Control Program (NSSCP) by Schistosomiasis Control Initiative (SCI) in 2005 and 2006 supported by Bill and Melinda Gates Foundation [5]. These programmes were only targeting school-aged children using MDA. This planned 5-year control programme that unfortunately had just lasted for 2 years was under coordination of Ministry of Health and Social welfare (MoHSW) and Ministry of Education and Vocational Training (MoEVT), Tanzania. Since then there have been no control programmes in the study areas before implementation of the current integrated control programme.
Study design and sampling procedureThis was a rather before-after intervention study design involving all inhabitants on Kome Island. After the baseline survey in February 2009 before implementation of any interventions, each round of MDA interventions was evaluated by follow-up parasitological and morbidity surveys which were conducted almost annually for impact evaluation, where the last fifth survey was performed in July 2013.
Before the surveys and intervention implementation, meetings were held in each village and primary schools where the purpose of study was explained. About 50 adult participants each from 8 villages (all villages in Kome Island) where, in primary schools, about 150-200 children each for annual crosssectional surveys have been randomly selected and recruited for participating in these follow-up cross-sectional surveys.
Mass drug administrationMDA is among the important control strategies that have been implemented in Kome island for morbidity control. All community members (≥4 years) were administered with PZQ and ALB. Only ALB was administered to children aged 2-4 years. Before MDA exercise, all community members were instructed to take adequate meal before coming for taking drugs. For schoolchildren at schools, meals preparation and MDA were carried out at the primary/secondary school premises. During the initial 3 rounds of annual MDA (2009, 2010, and 2011), exercise was rather health service-based delivery where health care providers in assistant with village health workers and research teams from GNI and NIMR-Mwanza administered the drugs. However, this approach was found to be much costly and unlikely to be sustainable; therefore the rest other 2 rounds of MDA were delivered through community-direct treatment (CDT), where trained community drug distributors were responsible for administering drugs (PZQ and ALB) among their fellow adult community members. For schoolchildren at schools, trained primary school teachers (1 male and 1 female in each school) administered drugs. During implementation of CDT and school-based delivery, close monitoring by health care personnel was ensured for managing side effects. The treatment coverage of estimated population of about 40,000 was based on extrapolated data of 2002 national census. Before the first MDA round in 2009, extrapolated population estimate were verified by conducted enumerated censor (39,895) and found to be almost the same as the national censor.
The fifth round of MDA using PZQ and ALB have been carried out among all eligible community members (≥2 years); the first one was in May 2009, followed by the other 4 consecutive annual MDA and their respective treatment coverage (Fig. 1). The fourth and fifth rounds of MDA using CDT improved remarkably the treatment coverage from 42.6% (of previous third round) to 69.4% and 76.8%, respectively.
Health education using PHAST approachHealth education was also applied for integrated schistosomiasis and STHs control. Implementation of PHAST started after the main training workshop in June-July 2010 that was followed by refresher training workshops among CORPS; the first one was between January and February 2011 and the second one in August 2012. This activity took about 18 months where at least 1 adult family member (gender balance alternating male and female) from each household had an opportunity of undergoing this training. While implementing PHAST, monitoring and supervision of CORPS who are responsible for facilitating PHAST strategy to their fellow villagers on the island have been going on. Similar kinds of health education had been planned but unfortunately it was not possible. However, such package of health education targeting schoolchildren have been considered during the 2nd phase among the integrated strategies for sustaining control of STHs on Kome Island.
To have good understanding of community perceptions and attitude towards schistosomiasis and STHs and thus be in better position of developing relevant health intervention packages, survey on knowledge, attitude, and practices (KAP) were conducted among adults and school-aged children on Kome Island in November 2009 before introducing health education. After implementation of PHAST health education, a similar follow-up survey was also conducted among the same individuals during December 2012, who participated in the baseline survey. The detail of this survey and implementation has been explained elsewhere [14].
Provision of alternative safe water supplyThis was among the control strategies particularly for schistosomiasis control at least to minimize the lake water activities associated with the risk of acquiring schistosome infection. To ensure all inhabitants to have alternative easy access to safe water supply, construction of pump wells were planned at least 1 pumped well for each sub-village. The construction started in late 2009, and by mid 2011 almost a half (n=23) were completed.
The initial plan was to construct a shallow well, which unfortunately could not produce water throughout all seasons in the year. Out of 23 pumped wells, only 5 were able to produce water throughout the year, the other 12 were just seasonal (i.e., produce water during the rainy season only), and the other 6 were completely dry most of the time. To overcome this, efforts of drilling deep well were made but with no much success; only 4 out of 10 wells were able to produce water. Apart from the effort of GNI for drilling deep wells, another fellow donor (JAICA, Japan) had similar outcome; 8 out of 22 attempted deeper drilled wells were able to produce water. Thus, in total there were 17 pumped wells with water available throughout the year. After the futile attempt of getting deeper dilled wells, there was an attempt for looking for other possibility of getting pumped water from the Lake which also was found unfeasible. After the 1st phase of 5 years, continual construction of shallow pumped wells was resorted where further 25 shallow pumped wells have also been constructed. At the moment overall, there are about 48 working pumped wells.
Data collectionParticipants were given container to collect stool specimens; only a single stool sample was collected from pupils and adult community members. Collected stools were processed in duplicate by the Kato-Katz techniques for microscopic examination of S. mansoni and STH (hookworms, 3, and Trichuris trichiura) eggs. The mean intensity (S. mansoni and hookworms) was determined by taking the average number of eggs in both slides and then multiply by 24 to obtain eggs per gram (epg) of feces. To test for quality control, 10% of the slides were reexamined by supervisors (Korean Research Team).
Data handling and analysisData entry was done using CSpro (Borland International, Scotts Valley, California, USA) or EXCEL and a double entry system was used for quality control. Data was transferred to STATA version 8 software (Statacorp 2000, College Station, Texas, USA) for analysis. Analysis was done by generation of some frequency tables, cross tabulations, and calculation of the prevalence. The mean egg count for S. mansoni and hookworms was determined using the arithmetic means. A nonparametric test for trend was performed to test for a trend of decreased prevalence of S. mansoni and hookworms with time (months/years).
RESULTSAs shown in Fig. 2, there has been a consistent decline in the prevalence of S. mansoni and hookworm infection among schoolchildren with time from February 2009 to July 2012. This trend, declining in the prevalence of S. mansoni and hookworms with increasing in years from 2009 up to 2012, was modestly significant for both; S. mansoni (non-parametric test for trend: z=-1.73, P=0.083), and for hookworms non-parametric test for trend: z=-1.73, P=0.083 (Fig. 2). By the third follow-up survey in July 2012, S. mansoni prevalence among schoolchildren was reduced from 42.2% at the baseline in February 2009 to 4.0% (P=0.0000) in July 2013, with an overall reduction of 90.5%. The corresponding reduction for hookworms was from 20.8% to 1.4% (P=0.0000) with overall reduction of 93.3%. Even in the fifth survey in July 2013 as compared to the previous fourth follow-up survey done on January 2013, S. mansoni infection rate dropped from 9.7% to 6.7% and hookworms from 4.2% to 3.0%. Almost similar trends were also observed for the intensity of infection (Fig. 3). The intensity of S. mansoni infection was reduced from 181.2 epg in February 2009 to 92.7 epg in January 2013, a reduction rate of 48.8% while the corresponding reduction for hookworm intensity of infection within the same period was from 281.7 epg to 106.8 epg, with 62.1%. The remarkably decline in the intensity of infection was more marked for hookworms than S. mansoni, but declining trend in the intensity of infection for S. mansoni from February 2009 to February 2013 were modestly significant (non-parametric test for trend: z=-1.73, P=0.083).
As for adults, similar trends of steady decline in the prevalence of S. mansoni and hookworm infection (Fig. 4) were also noted, particularly from 2009 to 2012. A similar significant declining trend was observed for the prevalence of S. mansoni (z=2, P=0.046 ) from February 2009 to February 2013, and of hookworms from July 2010 to July 2013 (z=2, P=0.046) (Fig. 4). Among adults significant reduction in the prevalence was also noted from baseline and the third follow-up survey; S. mansoni prevalence was reduced from 31.7% to 5.3% (P=0.0000), with an overall reduction of 83.2%. The corresponding reduction for hookworms was from 16.7% to 7.2% (P=0.0001) with overall reduction of 56.9%. However, a remarkable steep decline in the infection prevalence was much more observed for S. mansoni from February 2009 to July 2012. There was no consistent (i.e., unsteady) downward decline in the intensity of infection of S. mansoni and hookworms (Fig. 5). From 2009 to 2012, there was a tendency of declining which was not even significant (non-parametric test for trend: z=-1.41, P=0.157), while thereafter from 2012 to 2013 a trend was rather increasing but not significant as well (non-parametric test for trend: z=-1.41, P=0.157).
Other STHs occurred at a very low prevalence during the baseline survey as follows: T. trichiura (2.0%), A. lumbricoides (0.6%), and Strongyloides stercoralis (0.1%) among schoolchildren while in adults they were T. trichiura (1.8%), A. lumbricoides (0.5%), and S. stercoralis (0.5%). Despite that low prevalence of other STHs, by July 2012 the rates were also markedly reduced; T. trichiura to 0.25%, A. lumbricoides to 0.1%, and S. stercoralis to 0.0%. Thereafter, in the follow-up survey, the prevalence of other STHs remained at that low levels; T. trichiura (0.5%) and A. lumbricoides (0.1%) in children and T. trichiura (0.4%) and A. lumbricoides (0.2%) in adults in January 2013 survey.
DISCUSSIONIntegrated control of schistosomiasis and STHs has contributed to a significant reduction of the prevalence of S. mansoni and hookworms on Kome Island and kept their prevalence low at a sustainable control level. S. mansoni and hookworm infection rate in schoolchildren and adults had been maintained at a low sustainable level (5-10%) from July 2012 to July 2013, after 5 rounds of MDA. During that period (2009 to 20012), the intensity of S. mansoni and hookworm infection among adults and schoolchildren showed a tendency of decrease after 3 rounds of MDA. Apart from MDA intervention, the decline to such a sustainable control level might also be attributed to other integrated control strategies implemented in this integrated control programme.
Schistosomiasis and STHs during the baseline survey and subsequent cross-sectional follow-up surveys showed a remarkably steady decline in the infection rate particularly from 2009 to 2012, and thereafter remained low at a sustainable control level. This finding is in line with previous studies [9,10,15-17] which showed remarkable declines with integrated control strategies. Similar success of integrated strategies was noted in epidemiological studies done among a community in Brazil [15] for S. mansoni and another in China for Schistosoma japonicum [16] in areas where chemotherapy was complemented with other control strategies such as health education, improved sanitation, and water supply. Similarly, a successful use of integrated control strategies against STHs has also been reported from systematic analysis by sanitation [9], and improvements in water, sanitation, and hygiene (WASH) [10]. Our results were in accordance with these studies, suggesting that integrated control strategies are highly effective in reducing schistosomiasis and STHs in a Lake Victoria island and onshore communities. Currently there is ongoing national NTD control programme mainly targeting school-age children in the lake zone focusing on schistosomiasis and STHs. To make this ongoing control programme a long term sustainable control particularly in the lake zone, programs supplementing with clean water supply, adequate sanitation, and health education are essential.
The slow decline after the 4th and 5th follow-up survey, compared with steep decline after the 3rd follow-up survey might be due to the delay in taking off other control strategy (health education-PHAST and supply of alternative source of water) and declining treatment coverage (from 62.8% in the first round to 42.6% in the 3rd round). However, introducing CDT, the treatment coverage improved to a justifiable level (70-75%). Implementation of PHAST started around early 2011 and by mid 2011, and about a half of targeted pumped wells were constructed. Unfortunately of those only about one third of them were producing water throughout all seasons. Another limitation of health education in the control programme probably was an insufficient coverage because it was not extended to schoolchildren as had been initially planned. Action-oriented health education was initially planned at primary schools, but was not possible due to limited resources. Instead, we opted simple health education using printed materials (leaflets and posters) as our main educational materials. Action-oriented health education has been found to be more effective in enhancing knowledge and changing behaviors among school-age children [12,13].
So far this is the first large scale integration control project of schistosomiasis and STHs covering such a large population in Tanzania particularly for S. mansoni in Lake Victoria, in such an integrated manner with MDA along with health education and improved water supply [7,18]. Previous integrated control programmes in Lake Victoria have been mainly in a small scale covering only a small population [2]. Lower rate of implementation of integrated control strategies in our local setting may be attributed to its challenges in the implementation. Effective control of intestinal schistosomiasis along Lake Victoria is very tricky and challenging, as all community members of all age groups are likely to be equally affected as consequence of unavoidable lake water contact activities and thus require control strategies targeting all community members.
Resources for transmission control such as provision of safe water and sanitary facilities in countries with poor resources in sub-Saharan Africa like Tanzania are to some extent difficult to find due to poor infrastructure and inadequate resources in these countries. Other constraint is behavior on lake water contact [15]. The impact of health education on behavior is determined by culture and takes time through a long term implementation to have an impact. In this control programme, an attempt of integrating health education control strategy to minimize water contact-related behavior was made. Unfortunately, in our intervention, all control strategies in integrated control programmes were not able to start simultaneously; the one started a little bit late (i.e. PHAST) and the other was incomplete (i.e. constructed pumped wells). In case these control strategies had started earlier and completed as anticipated, probably the impact on schistosomiasis and STHs control would have been much higher than observed.
Although chemotherapy has been the backbone of previous control programmes and remain an important control strategy for morbidity control [6,7]; however, dependence on chemotherapy alone is not sustainable. Complementing MDA with other preventive measures, focused on clean water, adequate sanitation, and health education are essential features of any long-term strategy for control and elimination of schistosomiasis. Recently, there are also an increase in emphasis by WHO [7] and other workers [18] on the role of health education, safe and adequate water supply, and sanitation to complement chemotherapy as an option for sustainable long-term effects on morbidity control. Increased effort for development in sanitation and safe water supply also has been under support of various world forum/summit held. Millennium Development Goals by United Nations established in September 2000 and the World Summit on Sustainable Development [19] held in September 2002, and during the 3rd World Water Forum [20] convened in March 2003 are among those of few efforts. The specific provision of these forums is to halve the number of people without access to clean water supply and sanitation by 2015.
The decline of mean intensity was unsteady and showed a tendency of increasing during consecutive cross-sectional follow-up surveys (February 2013 and July 2013). Such an inconsistent trend might be due to the methodology applied in determining the mean egg intensity. We applied arithmetic means for only those who were positive. Probably the majority of those positive cases are more likely those individuals evaded MDA or were absent during the previous rounds of MDA exercise. In addition, Kome inhabitants are highly mobile population as noted previously [21] in most other islands in Lake Victoria. This explanation might be true for the surge up in infection rate and intensity of infection of S. mansoni and hookworms in the 4th and 5th follow-ups particularly among adults. During the final stage, awareness of such a mobile population on our programme was much higher and therefore many of them were rushing toward to get our health services including laboratory/ultrasound examinations.
The prevalence shown in our study is likely to be lower than the actual one due to the methodology of a single stool sample collection. Single stool sample collection is unlikely to detect a light infection and thus underestimates the prevalence. Examination of a single stool sample is less sensitive and may not reflect the worm burden adequately [22]. However, such underestimation is likely to be consistent as parasitological testing procedure was the same throughout all 6 surveys in this study.
The treatment coverage was not optimally enough (at least 75% and above) as was anticipated. This might be attributed mainly to the high mobility of Kome inhabitants as had been noted by others [21] in most islands in Lake Victoria. The majority of inhabitants on Kome Island are mobile population involved with fishing activities to move to small islands and other related business during ‘Dagaas’ (R. argentous) fishing seasons. Many settlements in trade center are unstable and temporary. Many people outside Kome Island come to the trade centers during the fishing season. The high movement rate outside of Kome Island of inhabitants might also have partly contributed to the reduction of fish stock (especially Nile perch and Tilapia) [23] which is currently under threat; thus leading to poverty in these fishing communities. Other likely contributing factor for the lower treatment coverage is the method of health-based delivery of MDA during the previous 3 rounds of MDA; this approach was not ideal to attract clients for MDA. Mobile individuals are not likely to be around during MDA exercise. The CDT intervention strategy in which communities themselves direct the planning and implementation of intervention delivery has been successful for the annual distribution of ivermectin for controlling onchocerciasis in more than 19 African countries [24].
Despite challenges in implementation of integrated strategies, significant impact on reduction of S. mansoni and STHs (particularly hookworms) was achieved; the activities have successfully reduced S. mansoni and STHs infection status on Kome Island. If resources allowed, chemotherapy is highly recommended at least to be supplemented with one or more control strategies including health education, water supply, sanitation, and focal intermediate host snail control where is feasible. This study further suggests that monitoring and evaluation is a crucial component of any large-scale STHs and schistosomiasis intervention programme as also recommended by WHO [25]. Now the challenges ahead, which other control strategies should be continued to be implemented on Kome Island to maintain schistosomiasis and STHs at such sustainable control? To achieve that goal, control strategies including MDA using CDT approach either annually or at least biannually, continuation of health education among schoolchildren, some other activities such as sports and drama that focus on schistosomiasis and STHs, and construction of latrines to schools without such facilities, should be continued on this island.
ACKNOWLEDGEMENTSWe are grateful to the staff of National Institute of Medical Research, Mwanza, Tanzania for their assistance in doing this survey. Village leaders of Kome Island and school teachers are also thanked for their help in performing this survey.
REFERENCES1. McCullough FS. The distribution of Schistosoma mansoni and Schistosoma haematobium in East Africa. Trop Geogr Med 1972;24:199-207.
2. Odongo-Aginya EI, Doehring M, Lakwo TL, Etyono S, Luyinda LB, Roth J, Doehring E. Integrated control trial of schistosomiasis at Nakiwogo fishing village near Entebbe, Uganda. East African Med J 1996;73:495-498.
3. The United Republic of Tanzania. Control of Neglected Diseases in Ukerewe District. Collaboration Research Project between Imperial College and NIMR-Mwanza. Sponsored Project by Bill and Melinda Gates Foundation through SCI. 2007.
4. World Health Organization. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drug in Control Intervention: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland. WHO. 2006.
5. The United Republic of Tanzania. Tanzania Mainland-Ministry of Health. Control of Schistosomiasis and Soil-Transmitted Helminthes. Sponsored Project by Bill and Melinda Gates Foundation through SCI. 2004.
6. Savioli L, Renganathan A, Montresor A, Davis A, Behbahani K. Control of schistosomiasis: a global picture. Parasitol Today 1997;13:444-448.
7. World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helmiathiasis. Third report of the WHO Expert Committee. WHO Tech Rep Ser No. 912. Geneva, Switzerland. WHO. 2002.
8. Jia TW, Melville S, Utzinger J, King CH, Zhou XN. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis 2012;6:e1621.
9. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-anlaysis. PLoS Med 2012;9:e1001162.
10. Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med 2014;e1001620. doi: 10.1371/journal.pmed.1001620
11. Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop 2003;86:2283-2294.
12. Lansdown R, Ledward A, Hall A, Issae W, Yona E, Mafulu J, Mweta M, Kihamia C, Nyandindi U, Bundy D. Schistosomiasis, helminth infection and health education in Tanzania: achieving behavior change in primary schools. Health Educ Res 2002;17:425-433.
13. Mwanga JR, Jensen BB, Magnussen P, Aagaard-Hansen J. School children as health change agents in Magu, Tanzania: a feasibility study. Health Promot Int 2008;23:16-23.
14. Mwanga JR, Kaatano GM, Siza J, Chang SY, Ko Y, Cyril MK, Nsabo J, Eom KS, Yong TS, Chai JY, Min DY, Rim HJ, Changalucha JM. Improved perceptions and practices related to schistosomiasis and intestinal worms infections following PHAST intervention on Kome Island, North-Western Tanzania. Korean J Parasitol 2015;53:(in press).
15. Uchoa E, Barreto SM, Firmo JO, Guerra HL, Pimenta FG Jr, Kima e Costa MF. The control of schistosomiasis in Brazil: an ethnoepidemiological study of the effectiveness of a community mobilization program for health education. Soc Sci Med 2000;51:1529-1541.
16. Xu J, Xu JF, Li SZ, Zhang LJ, Wang Q, Zhu HH, Zhou XN. Integrated control programmes for schistosomiasis and other helminth infections in P.R. China. Acta Trop 2015;141:332-341.
17. Freeman MC, Clasen T, Brooker SJ, Akoko DO, Rheingans R. The impact of a school-based hygiene, water quality and sanitation intervention on soil-transmitted helminth reinfection: a cluster-randomized trial. Am J Trop Med Hyg 2013;89:875-883.
18. Utzinger J, Bergquist R, Xiao SH, Singer BH, Tanner M. Sustainable schistosomiasis control-the way forward. Lancet 2003;362:1932-1934.
19. WEHAB Working Group. A framework for action on water and sanitation. 2002.
21. Appleton J. ‘At my age I should be sitting under that tree’: the impact of AIDS on Tanzanian lakeshore communities. Gend Dev 2000;8:19-27.
22. Kongs A, Marks G, Verlé P, Van der Stuyft P. The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop Med Int Health 2001;6:163-169.
23. Muli JR. An Appraisal of stocking and introduction of fish in Lake Victoria (East Africa). In Cowx IG ed, Stocking and Introduction of Fish. Fishing News Books. Osney Mead, Oxford, UK. A Division of Blackwell Science Ltd. 1998, pp 258-265.
24. Homeida M, Braide E, Elhassan E, Amazigo UV, Liese B, Benton B, Noma M, Etya'alé D, Dadzie KY, Kale OO, Sékétéli A. APOC's strategy of community-directed treatment with ivermectin (CDTI) and its potential for providing additional health services to the poorest populations. African Programme for Onchocerciasis Control. Ann Trop Med Parasitol 2002;96(suppl 1):S93-S104.
25. Montresor A, Gyorkos TW, Crompton DWT, Bundy DAP, Savioli L. Monitoring helminth control programmes: guidelines for monitoring the impact of control programmes aimed at reducing morbidity caused by soil-transmitted helminths and schistosomes, with particular reference to school-age children. Geneva, Switzerland. World Health Organization. 1999.
Fig. 1.Annual treatment coverage (PZQ & ALB) of eligible community members (≥ 4 years) over 5-year period. 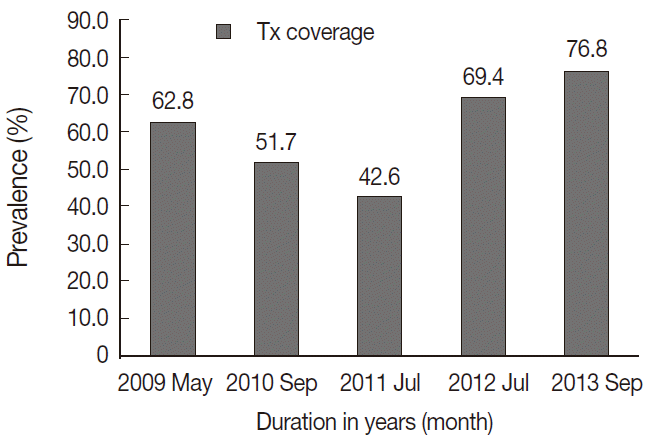
Fig. 2.Trends of prevalence of S. mansoni and hookworms among schoolchildren on Kome Island over 5 years. S.m=S. mansoni; Hw=hookworms. 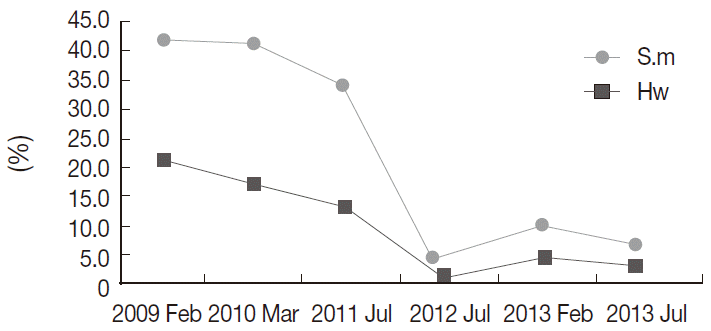
Fig. 3.Trends of mean intensity of infection with S. mansoni and hookworms over 5 years among schoolchildren on Kome island. S.m=S. mansoni; Hw=hookworms. 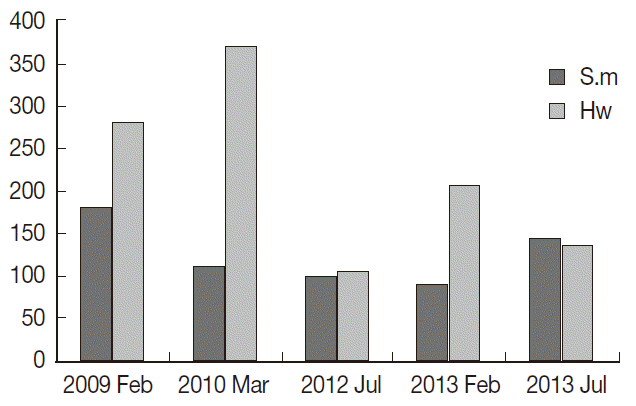
|
|
|||||||||||||||||||||||||||||||||||||||||