AbstractIn July 2014, a nematode species, Schistorophus cirripedesmi Rhizhikov and Khokhlova, 1964, was recovered from a bar-tailed godwit, Limosa lapponica baueri that was stored in a -20˚C freezer in the Chungnam Wild Animal Rescue Center. The bird was collected in 2012 from the coastal region of Pyeongtaek-si (City), Gyeonggi-do (Province) in the Republic of Korea, although the exact date is not clear. At necropsy, 9 nematodes were found in the gizzard of the bird. The parasites had 4 horn-like cephalic cuticular ornamentations. After morphometric comparison and morphological observations, including scanning electron microscopy, the nematodes were identified as S. cirripedesmi. This is the first description of a nematode species in a shorebird in Korea. This is also the first time this genus and species have been found in Korea.
The coastal regions of Korea, especially the Yellow Sea, are important staging or breeding sites for a variety of shorebirds. To date, 63 species belonging to 7 families (Haematopodidae, Recurvirostridae, Charadriidae, Rostratulidae, Jacanidae, Scolopacidae, and Glareolidae) have been recorded in Korea [1]. Considering the diversity and numbers of these birds, it is likely that they bear many different species of parasites. However, relatively little is known about the parasite fauna in the shorebirds in Korea: to date, only 8 species of parasites in 9 species of shorebirds have been recorded in Korea [2-7]. To the best of our knowledge, other helminths such as nematodes or cestodes have never been reported in shorebirds in Korea.
The bar-tailed godwit, Limosa lapponica (Linnaeus, 1758), is a shorebird that visits the Yellow Sea region frequently. It is a relatively large wader species that is renowned for its extreme migration. It spends time in Northern Eurasia and Western Alaska to breed and then migrates to its wintering locations, namely, Europe, Africa, Middle and South Eastern Asia, and Australia. It has several subspecies. Of these, L. lapponica baueri and L. lapponica menzbieri can be observed in Korea during the migrating seasons, which are early April to mid-May and early August to early October [1]. According to the reports on its migration, the subspecies L. l. baueri often chooses a circuitous route during its northward migration that includes the Yellow Sea region, Japan, Kamchatka Peninsula, and the Bering Islands as its staging regions. After completing its breeding in Alaska, it returns to New Zealand directly or via a circuitous route that follows coastal regions [8-10].
In the present study, we report for the first time a nematode species, Schistorophus cirripedesmi Rhižhikov and Khokhlova, 1964, in a shorebird in Korea. The shorebird was a L. lapponica baueri that had been rescued in Korea but then died and was stored in a -20˚C freezer in the Chungnam Wild Animal Rescue Center (CWARC). The parasitic faunas of migratory birds in Korea are discussed.
A bar-tailed godwit was rescued from the coastal region of Pyeongtaek-si (City), Gyeonggi-do (Province) in the Republic of Korea (=Korea) in 2012, although the exact date is not clear. The bird was an adult and died during treatment. Its visceral organs were stored in a -20˚C freezer in the CWARC and then donated in July 2014 to the Parasite Resource Bank in Korea (PRB) for parasitological examinations. At necropsy, 9 nematodes (4 females and 5 males) were found in the wall of the gizzard (Fig. 1). Their anterior extremity was located at the muscular layer of the organ. Three female and 3 male worms were chosen for morphological identification, and slide specimens were generated using glycerin-jelly. The specimens were placed under a light microscope, and all organs were measured with a micrometer. Two of the specimens studied are deposited in the National Institute of Biological Resources in Korea (NIBR). NIBR specimen numbers are KOSPIV0000221892 and KOSPIV0000221893. A male and a female worm were subjected to scanning electron microscopy (SEM). Thus, they were fixed in 2.5% glutaraldehyde after washing in PBS 3 times, each for 15 min. Thereafter, they were dehydrated using a graded alcohol series (70%, 80%, 90%, 95%, 100%, and again 100% ethyl alcohol; 30 min per step). The specimens were then dipped in hexamethyldisilazan for 1 hr, after which they were dehydrated again in a sealed box with silica gel for 1 day. After coating with gold, they were observed by SEM (LEO-15301; Oberkochen, Germany).
The light microscopy and SEM analyses indicated that the body was thin and long. The head was compressed laterally. The oral opening was narrow dorsoventrally and surrounded by 2 pairs of teeth on each side. The pseudolabia were well developed, square shaped, and covered the lateral part of the head. Two pairs of cephalic papillae were located on each side of the pseudolabia. An amphid was observed at the extremity of each pseudolabia. Four sublabia were situated at the sublateral part of the pseudolabia and elongated to the posterior extremity of the pseudolabia. Four ptilina originated from the lower position of each sublabium. The ptilina were well developed and resembled a hawk’s wing (Fig. 2A-D). The buccal cavity was well developed, deep, and narrow (Fig. 3A). The esophagus was divided into a muscular and a glandular esophagus. The muscular esophagus was relatively shorter than the glandular esophagus. The deirid was very small and shaped like a dragon fruit (Fig. 2C, E). Under light microscopy, its location was obscure. Table 1 presents the morphometric measurements of male and female worms (Table 1).
The posterior part of the male worm was coiled. The caudal alae were well developed. The tail was short and had a blunted end. The papillae were located on the ventral side of the caudal alae. There were 20-21 pairs of preanal papillae and 4 pairs of postanal papillae (Figs. 3D, 4A). There were 2 spicules that were unequal in size and dissimilar. The left spicule was long and slender. Its distal end was complicated with several processes (Fig. 4B). The right spicule was stout, short, and curved with a triangular process slightly ventrally near distal end (Fig. 4C).
The female worm was much larger than the males in terms of both length and width. The vulva was prominent, was located almost in the middle of the body (Figs. 2F, 3B). The uterus was didelphic and had an amphidelphic position. It was packed with numerous eggs. The vagina vera and vagina uterine could not be observed well. The eggs near the vulva were larger than the other eggs and were embryonated. The eggs (n=30) were thick-shelled. The tail was short and had a blunted end (Fig. 3C).
The family Acuariidae is characterized by cuticular ornamentation of the anterior extremity. In terms of the species in this family, the species in the genus Schistorophus Railliet, 1916 are easily distinguishable from those in other genera because of the presence of 4 characteristic horn-shaped cephalic cuticular ornamentations [11]. In the past, 16 species of the genus Schistorophus were described. However, Wong and Lankester [12] revised the genus after observing the specimens recovered from North American waders and burrowing specimens. They recognized only 5 valid species, Schistorophus longicornis (Hemprich and Ehrenberg, 1866) Railliet, 1916; S. skrjabini (Vassilkova, 1926) Guschanskaya, 1950; S. cornutus Sobolev, 1943; S. guschanskoi Ablasov and Chibichenko, 1962, and S. cirripedesmi Rhizhikov and Khokhlova, 1964 [12]. Zhang [13] added 1 more species, S. fukienensis, which was found in China, and Pinto et al. [14] described the 7th species, S. molini.
Wong and Lankester [12] proposed the term ‘ptilinum’ for the cephalic horn-like structures of the genus Schistorophus and used the size of the ptilinum as one of the keys for distinguishing between the species of the genus. They also used the length and shape of the spicules and the number of cloacal papillae [12]. Pinto et al. [14] modified this species key and added S. molini as a species. The specimens in the present study undoubtedly belong to the genus Schistorophus because of their ptilina. Moreover, the other characteristics in the species key proposed by Wong and Lankester [12] and Pinto et al. [14] indicate that our specimens belong to S. cirripedesmi, namely, the length of ptilina (105-125 μm in the male and 120-142.5 μm in the female), length of the left spicule (460-470 μm), and number of pre-cloacal papillae (20-21 pairs). The morphometric measurements of our specimens also matched well with those of the previous S. cirripedesmi specimens. Table 1 does not include the size of the undeveloped eggs, but, in our specimens, the eggs inside the early uterus were smaller than those near the vulva. Such size variation in the eggs was also evident in Fig. 1 of Rhizhikov and Khokhlova, 1964, who described the first specimens of S. cirripedesmi [15].
When S. cirripedesmi was first described in Vietnam by Rhizhikov and Khokhlova, the host was Charadrius leschenaultia (Lesson, 1826) [15], although, in their original description, Rhizhikov and Khokhlova stated that the host was ‘Cirripedesmus leschenaulti’. It is likely that the genus name ‘Cirripedesmus’ was a miscitation of ‘Cirrepidesmus Bonaparte, 1856’ [16], which is regarded as a synonym of the genus Charadrius. Thus, the scientific name of the nematode species S. cirripedesmi appears to have originated from the wrong host genus name. This requires further discussion.
In terms of distribution, S. cirripedesmi has been recorded in both the Old World (China, Europe, Egypt, Malaysia, Russia, and Vietnam) and the New World (Antigua) [12,15,17-21]. However, there is some doubt about its New World distribution, which was recorded by Clapham [18]. He reported finding of nematode larvae belonging to the genus Schistorophus in the gizzard of a willet, Tringa semipalmata (=Catoptrophorus semipalmatus), and a killdeer, Charadrius vociferus (originally recorded as a rufous necked plover, Oxychus vociferus), in Antigua. Moreover, he recorded adults belonging to the genus Schistorophus in the gizzard of a black-necked stilt, Himantopus mexicanus [18]. Although Clapham [18] concluded on the basis of the morphology of these specimens that they were S. laciniatus, he mentioned that this identity was in some doubt because an adult male was not found [18]. Wong and Lankester [12] commented that the specimens described by Clapham [18] were identical to the description of S. cirripedesmi presented in their study and thus considered the specimens of Clapham to be S. cirripedesmi. However, the description of Clapham [18] was based on adult female worms only, and this description is also very similar to that of the female S. molini, which was recorded in Brazil [14]. Thus, because of the incomplete description, we cannot make a definite conclusion regarding the identity of Clapham’s specimens: the deposited specimens should be observed in detail to resolve this issue. At this point, it seems advisable to treat Clapham’s specimens as belonging to a species inquirendae, namely, S. laciniatus, as recorded by Clapham [18]. Thus, at this time, we tentatively conclude that the distribution of S. cirripedesmi may not include Antigua.
Nevertheless, there is some evidence that S. cirripedesmi is also distributed in Australia. Mawson [19] described the nematodes of waders in Australia. These included 2 different Schistorophus species, 1 in Calidris canutus and 1 in L. lapponica (possibly L. lapponica baueri). Wong and Lankester [12] discussed the worms found in L. lapponica by Mawson [19] and stated that they belonged to S. longicornis. However, they did not discuss the worms found in C. canutus [12]. Mawson [19] mentioned that the latter worms closely resembled the description of S. longicornis by Li [20] but differed from the record of S. longicornis by Akhtar. Interestingly, the specimens of Li [20] were originally considered to belong to S. longicornis but were later considered by Daiya, Bondarenko, and Gubanov to belong to S. lii [20,21]. Notably, Wong and Lankester [12] considered S. lii to be a conspecific species with S. cirripedesmi. In addition, the drawings provided by Mawson [19] completely matched the description of S. cirripedesmi that was proposed by Wong and Lankester [12]. On this basis, we conclude that the distribution of S. cirripedesmi includes Australia.
Satellite tracking technology revealed the extreme migrations of L. lapponica baueri, the shorebird host of the specimens described in the present study. These birds employ a wide range of Eastern Pacific regions for their airway. They fly directly from the non-breeding site of New Zealand to the Yellow Sea region; on average, they fly 10,060 km in 7.2 days [10]. Moreover, the flight from the Yellow Sea region to the breeding region in Alaska is 6,770 km, which is achieved on average in 4.6 days [10]. There is also a case of a female L. lapponica baueri that flew for the 29,280 km (including 11,680 km over 8.1 days) between Alaska and New Zealand during its entire migration [9,10]. She only landed at 3 spots: New Zealand, the Yellow Sea, and Alaska [10]. This extremely fast migration of the host indicates that unless the parasites are eliminated by their host before leaving, there is a high possibility that the host can carry the parasite from the point of departure to the destination: it is likely that if the lifespan of S. cirripedesmi is longer than few days it can take the birds to travel the long distances. Indeed, several reports state that L. lapponica taymyrensis that were caught in Castricum, the Netherlands, before reaching their stop-over site in the Wadden Sea had nematodes in their feces [22,23]. Thus, apprehending the route and duration of migration by the hosts of S. cirripedesmi will help us to better understand the distribution of the parasite. This was also suggested by Wong and Anderson [17]. Thus, as explained above, S. cirripedesmi has been reported in Korea (Yellow Sea), Kamchatka Peninsula, and Australia [19,21]; all of these places overlap with the migration route of L. lapponica baueri [8-10]. The existence of the species in stop-over sites indicates that they could be carried to breeding sites; thus, it seems likely that S. cirripedesmi will be present in L. lapponica baueri in New Zealand and Alaska as well. This insight may also be useful for understanding the distributions of other parasites. In particular, the cestodes Ophrycotyle proteus Friis, 1870 and Capsulata edenensis Sandeman, 1959 [24], and the acanthocephalans Profilicollis antarcticus and P. novaezealandensis [25] have been recorded in L. lapponica baueri in New Zealand, while the nematode, S. longicornis has been recorded in L. lapponica baueri in Australia [19]. This indicates that these parasites may also be distributed in Korea.
In conclusion, we believe that our specimens are S. cirripedesmi on the basis of morphological observations. This is the first time both the genus and species have been found to belong to the Korean parasite fauna. This is also the first time a nematode parasite has been recorded in shorebirds in Korea. While we sought to estimate the distribution of S. cirripedesmi by examining the migration of the host, many questions remain, including the location of infection, sources of infection, and host-parasite interactions. Further studies that examine these facets are needed to improve our understanding of the ecology of S. cirripedesmi.
ACKNOWLEDGEMENTSThis work was supported by a grant from the National Institute of Biological Resources (NIBR), which is funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR no. 2014-02-001). The parasite materials used in this study were provided by the Parasite Resource Bank of Korea of the National Research Resource Center (2012-0000037) of the Republic of Korea.
REFERENCES1. Park JY, Park JG, Choi CY. A photographic guide to the shorebirds of Korea. No. 5. Checklist of organisms in Korea. Nature and Ecology. Seoul, Korea. pp 1-348.
2. Chu JP, Cho YJ, Yoo JC, Park SG. Himasthla kusasigi (Trematoda, Echinostomatidae) recovered from the intestine of the Dunlin, Calidris alpine sakhalina, in Korea. Korean J Syst Zool 1987;16:125-131.
3. Ryang YS, Yoo JC, Lee SH, Chai JY. The palearctic oystercatcher Haematopus ostralegus, a natural definitive host for Gymnophalloides seoi. J Parasitol 2000;86:418-419.
4. Seo M, Guk SM, Chai JY. The ruddy turnstone, Arenaria interpres interpres, a new definitive host for Gynaecotyla squatarolae (Digenea: Microphallidae). Korean J Parasitol 2008;46:41-43.
5. Chung OS, Lee HJ, Sohn WM, Park YK, Chai JY, Seo M. Discovery of Parvatrema duboisi and Parvatrema homoeotecnum (Digenea: Gymnophallidae) from migratory birds in Korea. Korean J Parasitol 2010;48:271-274.
6. Chung OS, Sohn WM, Chai JY, Seo M, Lee HJ. Discovery of Maritrema obstipum (Digenea: Microphallidae) from migratory birds in Korea. Korean J Parasitol 2011;49:457-460.
7. Yoo HJ, Chung OS, Seo M. Discovery of Endocotyle incana and Spelotrema pseudogonotyla (Digenea: Microphallidae) from Scolopacid migratory birds in Korea. Korean J Parasitol 2012;50:273-276.
8. Riegen AC. Movements of banded arctic waders to and from New Zealand. Notornis 1999;46:123-142.
9. Gill RE Jr, Tibbitts TL, Douglas DC, Handel CM, Mulcahy DM, Gottschalck JC, Warnock N, McCaffery BJ, Battley PF, Piersma T. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc R Soc B 2009;276:447-457.
10. Battley PF, Warnock N, Tibbitts TL, Gill Jr RE, Piersma T, Hassell CJ, Douglas DC, Mulcahy DM, Gartell BD, Schuckard RS, Melville DS, Riegen AC. Contrasting extreme long-distance migration patterns in bar-tailed godwits Limosa lapponica. J Avian Biol 2012;43:1-12.
12. Wong PL, Lankester MW. Revision of the genus Schistorophus Railliet, 1916 (Nematoda: Acuarioidea). Canadian J Zool 1984;62:2527-2540.
13. Zhang L. Three new species of Acuarioidea from birds from Fujian, China. Sichuan J Zool 1987;9:1-4.
14. Pinto RM, Vicente JJ, Muniz-Pereira LC, Noronha D. On two nematodes from Brazilian birds and description of a new species (Acuarioidea, Schistorophinae) parasitizing Laterallus viridis (Müllet, 1776) (Gruiformes, Rallidae). Mem Inst Oswaldo Cruz (Rio de Janeiro) 1999;94:751-755.
15. Rhizhikov KM, Khokhlova IG. Two new nematode species Schistorophus cirripedesmi n. sp. and Viktorocara helcyoni n. sp. from wild birds in Vietnam. Helminthologia 1964;5:107-114.
16. Bonaparte CL. Excursions dans les divers musee d’Allemagne, de Hollande et de Belgique, et tableaux paralleliques de l’ordre des Echassiers. Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences 1856;43:410-421.
17. Wong PL, Anderson RC. Acuarioids (Nematoda: Acuarioidea) from waders and terns (Aves) in Sabah, East Malaysia: evidence for transmission on the wintering ground. Can J Zool 1984;63:1706-1710.
19. Mawson PM. Some Acuariinae (Nematoda) from Australian birds. Trans R Soc South Australia 1982;106:19-30.
20. Li HC. Report on a collection of parasitic nematodes, mainly from North China. Part 2. Spiruroidea. Trans Am Microscop Soc 1934;53:174-195.
21. Daiya GG, Bondarenko SK, Gubanov NM. Description of Schistorophus lii n. sp. and. S. longicornis (Hemprich and Ehrenberg, 1866). Sb Rab Gel’mintol Akad Skrj “KOLOS”. pp 118-122.
22. Landys MM, Piersma T, Visser GH, Jukema J, Wijker A. Water balance during real and simulated long-distance migratory flight in the bar-tailed godwit. Condor 2000;102:645-652.
23. Piersma T, Mendes L, Hennekens J, Ratiarison S, Groenewold S, Jukema J. Breeding plumage honestly signals likelihood of tapeworm infestation in females of a long-distance migrating shorebird, the bar-tailed godwit. Zoology 2001;104:41-48.
24. Schmidt GD, Allison FR. New records of cestodes of New Zealand birds. New Zealand J Zool 1989;16:465.
25. Brockerhoff AM, Smales LR. Profilicollis novaezelandensis n. sp. (Polymorphidae) and two other acanthocephalan parasites from shore birds (Haematopodidae and Scolopacidae) in New Zealand, with records of two species in intertidal crabs (Decapoda: Grapsidae and Ocypodidae). Syst Parasitol 2002;52:55-65.
Fig. 2.Scanning electron microscopic views of Schistorophus cirripedesmi. (A) Apical view of the head. (B) Lateral view. (C, D) Dorsal views. Arrow. deirid. (E) Enlarged view of the deirid that looks like dragon fruit. (F) Vulva opening. 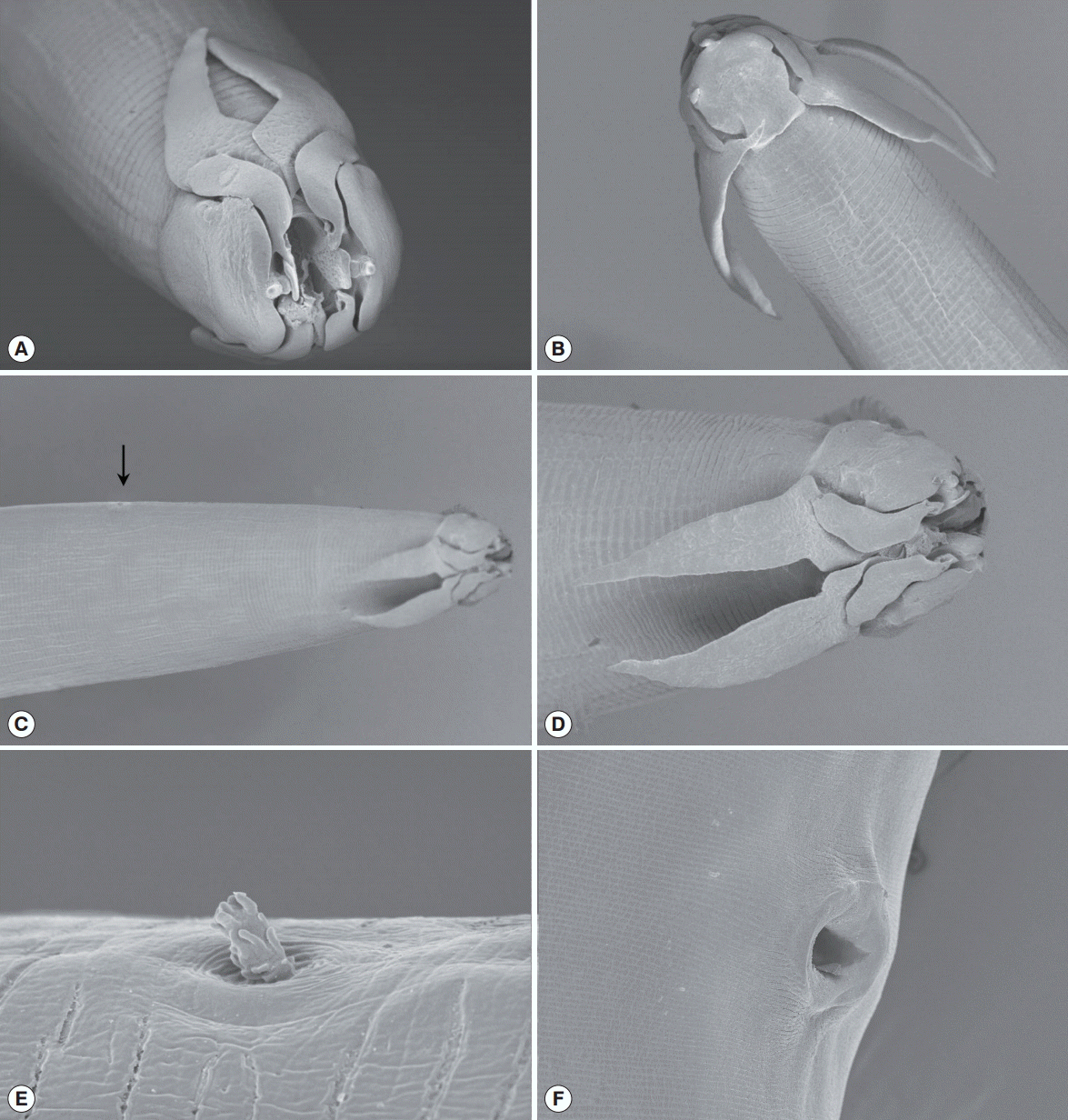
Fig. 3.Light microscopic views of Schistorophus cirripedemi attached. (A) Lateral view of a female worm. (B) Larvated eggs in the uterus and prominent lips of vulva. (C) Posterior extremity of a female worm. (D) Posterior extremity of a male worm. 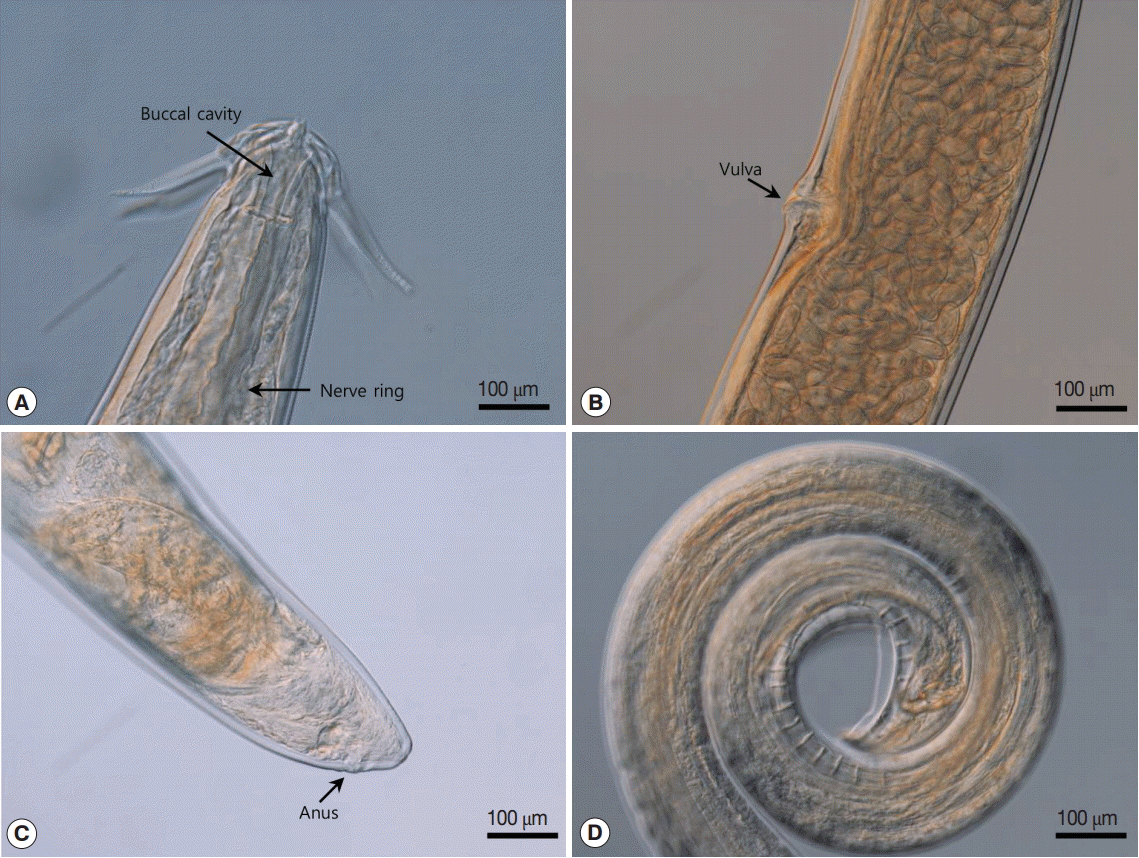
Fig. 4.(A) Posterior part of the male Schistorophus cirripedemi with papillae and spicules. (B) Left spicule. (C) Right spicule. 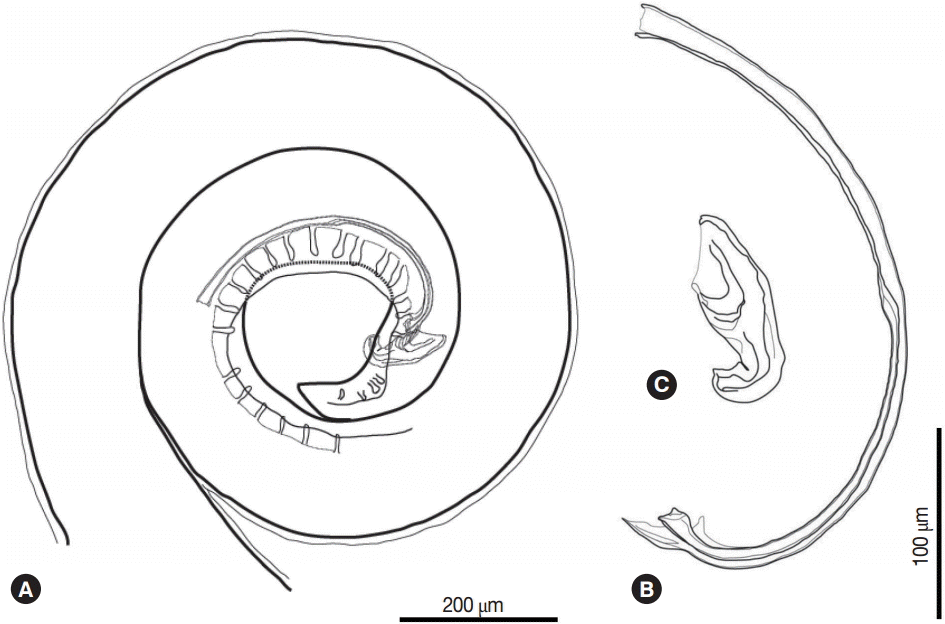
Table 1.Morphometirc comparison of previously described Schistorophus cirripedesmi and the present study
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||