INTRODUCTION
Acanthamoeba keratitis (AK) is an uncommon serious corneal infection caused by free-living protozoa in the genus Acanthamoeba. The infection routes are direct contact of corneal trauma with amoebae or exposure to contaminated dust, soil or water [1]. In developed countries, the infection frequently occurs in healthy individuals, especially in contact lens (CL) wearers with improper contact lens hygiene. However, in developing countries including Thailand, AK is a rare disease compared with other common keratitis caused by viruses, fungi and bacteria, and mostly reported from healthy hosts whose activities related to agricultural fields [1]. The incidence in developing countries is generally low, but this may be underestimation due to several reasons, such as little awareness of clinicians who are unfamiliar with the infection, or believing that AK is principally correlated to immunocompetent patients who wear CL, and lack of laboratory diagnostic capacity.
Symptoms for AK are not specific, however terrible eye pain is typically disproportionate to the degree of keratitis. Clinical signs such as epithelial defects or central ulcers, anterior stromal ring infiltrates and radial keratoneuritis are commonly present [2–4]. No significant differences in signs or symptoms were detected between contact and non-contact lens related patients [2]. Diagnosis for AK relies on in vivo confocal microscopy (IVCM) or detection of amoebae from corneal scrapings by staining or agar culture of which methods need skillful observers. As symptoms of AK are nonspecific and similarly to those presenting in other microbial keratitis, delayed diagnosis or misdiagnosis may lead to prolonged therapy which cause amoebae encystment and recurrent infection. Here, we report the successful detection and treatment of AK in a non-CL wearer with HIV infection from the tertiary eye care in Northern Thailand. This study was approved by the Research and Ethics Committee, Faculty of Medicine, Chiang Mai University (study code: OPT-2561-05965) and written consent was obtained from the patient.
CASE RECORD
A 31-year-old homosexual man with asymptomatic HIV infection had been treated at Lampang provincial hospital with severe pain, redness and photophobia of his left eye for about 3 weeks. The eye symptoms began with the foreign body sensation during motorbike riding and gradually became worse. He had neither history of HIV therapy nor experience of wearing CL. His recent CD4 count was 334 cells/mm3. No clinical manifestation of any opportunistic infections related to HIV infection was detected. Ocular examination revealed corneal ulceration with hypopyon. A sample of corneal scraping was then taken and sent for cultures before topical antifungal and fortified antibiotics were applied. However, the scraping cultures were negative for both bacteria and fungus. After 6-week treatment without clinical improvement, he was referred to Chiang Mai University (CMU) hospital which is a tertiary hospital in Northern Thailand.
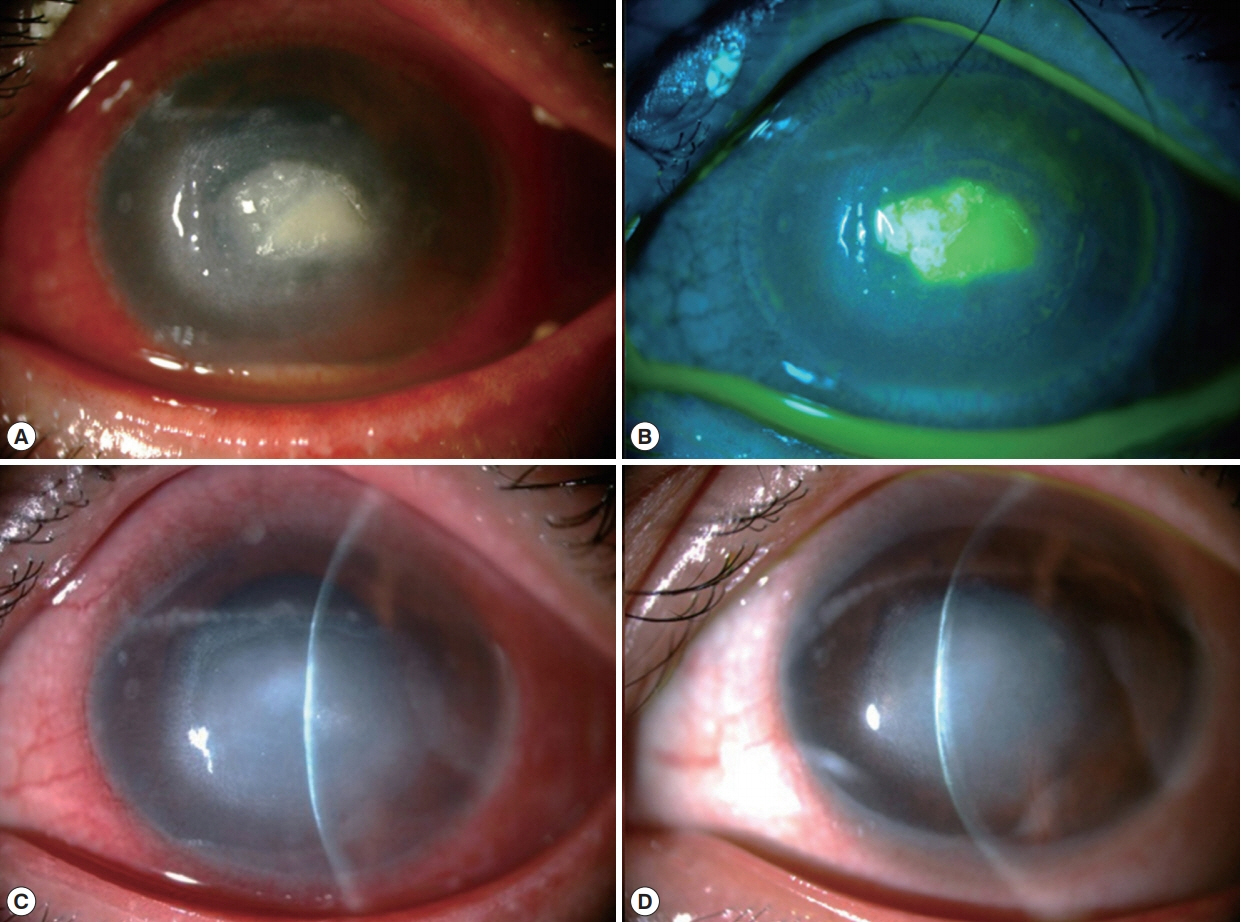
At CMU hospital, the visual acuity (VA) was 6/6 and hand motion in the right and left eye, respectively. Slit lamp biomicroscopy of the left eye revealed dense corneal stromal infiltration involved the visual axis with overlying epithelial defect, ring infiltration and multiple satellite lesions. There was pannus formation involved the inferior half of the cornea and a layer of hypopyon in the anterior chamber (Fig. 1A, B). The intraocular pressure and the posterior eye segment evaluation by B-scan ultrasonography in the affected eye were normal. Initially, corneal scraping was performed for microbial work-up and the specimens were subjected for microscopic exam with Gram’s stain, potassium hydroxide (KOH) wet mount, calcofluor white (CFW) stain and culture for bacteria and fungi. IVCM (Confoscan 4, Nidex Technologies, Italy) was also performed with negative results for neither fungal elements nor any microbial organisms. He was admitted and administrated with topical eye drops (5% natamycin hourly and 0.5% levofloxacin 2 hourly and 1% atropine 4 times/day) and oral itraconazole.
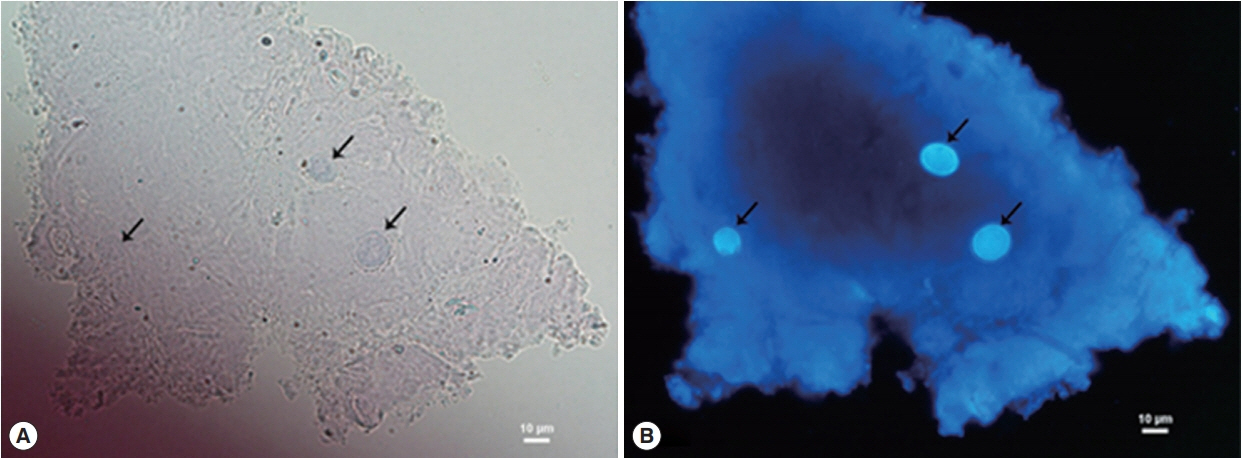
Three days after treatment, corneal conditions did not respond to the medications and the ring infiltration gradually progressed. The results of Gram’s stain, KOH and cultures for bacteria and fungus, were all negative, while Acanthamoeba-like cysts were found by CFW staining. The cysts were distinctively fluoresced as bright blue, particularly at inner cyst wall, which clearly contrasted with corneal epithelium background (Fig. 2).
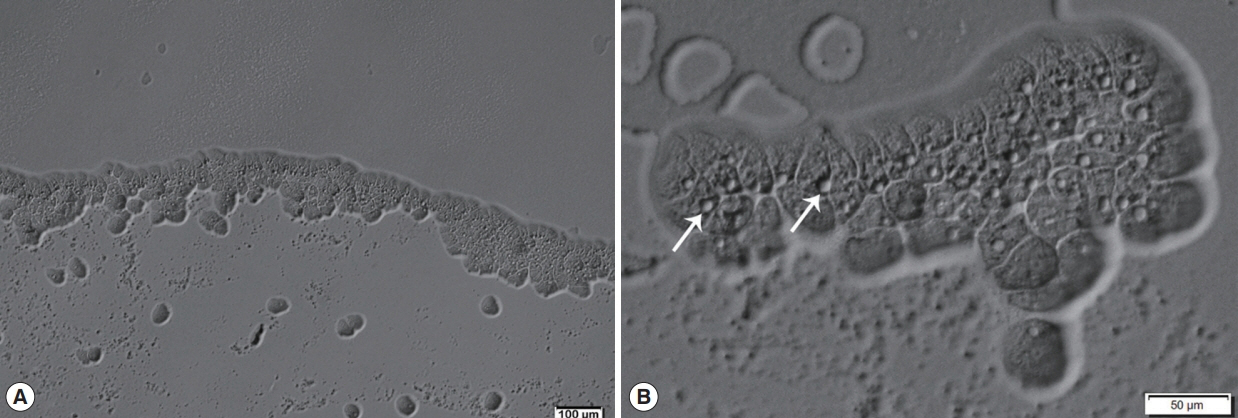
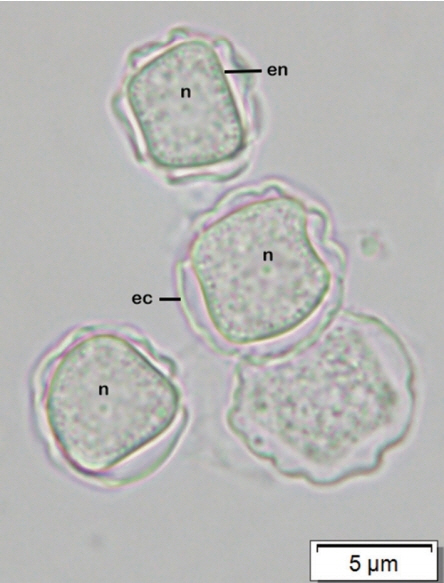
Afterward, a sample of corneal re-scraping was subjected to Acanthamoeba culture. Monoxenic culture was performed on a 1.5% non-nutrient agar plate pre-coated with heat-inactivated Escherichia coli as previously described [5]. On the second day of incubation, a clear plaque on the bacteria lawn resulting from bacterial consuming by Acanthamoeba was clearly seen. Direct observation under the light microscope, clusters of trophozoites were observed at circumferential outer rim of the plaque (Fig. 3). By wet smear preparation with saline, cysts of the isolate comprised a double-layered wall with the size less than 18 μm in diameter (Fig. 4), which are unique characteristics of the cysts in Acanthamoeba group II based on Pussard and Pons’ classification [6].
For molecular confirmation, genomic DNA from the Acanthamoeba isolate (called CMU_AK herein) was extracted using E.Z.N.A.® Tissue DNA Kit (Omega Biotek, Germany). The partial 18S ribosomal DNA was amplified by Acanthamoeba spp.-specific PCR (ACA PCR) using genus specific primers (JDP1/JDP2) as previously described [7]. An Acanthamoeba clinical isolate from Mahidol University (MU_AK) was used as a positive control in this study. The PCR products were purified using E.Z.N.A.® Cycle Pure Kit (Omega Biotek, Doraville, Georgia, USA) and consequently sent to First BASE Laboratories Sdn. Bhd. (Selangor, Malaysia) for Sanger sequencing. The purified amplicons were bidirectionally sequenced using the same primers as previously used in the PCR. Obtained sequences were manually edited and assembled using BioEdit software version 7.0.9.0 [8]. The partial 18S rDNA sequences (416 bp excluding primers) of the CMU_AK and the MU_AK were deposited in GenBank under accession numbers MH038174 and MH038175. The 2 sequences were very similar with 99.27% pairwise identity and only 3 nucleotide differences of transition mutation were detected. For sequence similarity searches, the sequence of CMU_AK was compared with sequences in the GenBank database using the BLAST tool available at www.ncbi.nlm.nih.gov. The BLAST result revealed that Acanthamoeba sp. T4 genotype from USA (JX423600) was the first sequence hit to that of the CMU_AK with 100% identity.
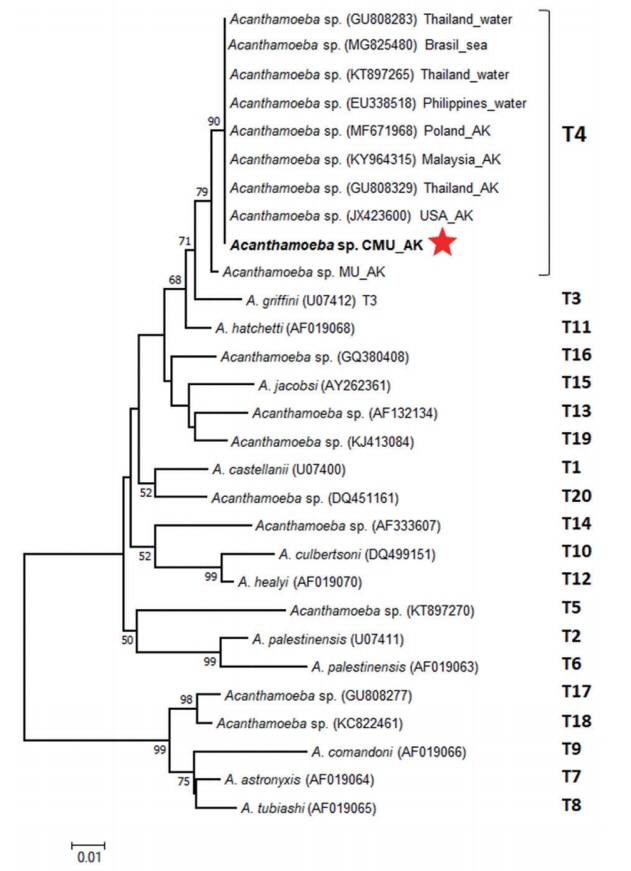
To classify Acanthamoeba genotype, neighbor-joining (NJ) phylogenetic tree based on the partial 18S rDNA sequence alignment of the CMU_AK, the MU_AK and 20 representative genotypes (T1–T20) accessible from the GenBank, was conducted through MEGA6 software [9] using the Kimura 2-parameter method [10] with 1,000 bootstrap replications. The tree topology presented 2 main distinct clades associated with the cyst morphology categorized by Pussard and Pons [6] (Fig. 5). The big clade comprised of the CMU_AK, the MU_AK and other Acanthamoeba references corresponding to Acanthamoeba group II or group III (cyst diameter <18 μm), while the small clade consisted of Acanthamoeba group I (cyst diameter >18 μm) [6,11]. Analyzed genotypes were clearly separated into their own monophyletic clade. The CMU_AK was clustered into T4 clade together with the MU_AK and other Acanthamoeba T4 references (Fig. 5).
After the causative agent was clarified, the patient was then treated with topical polyhexamethylene biguanide (PHMB) hourly. On the third day after treatment, the ring infiltration was resolved, therefore the frequency of PHMB application was gradually reduced to 2 hourly and the patient was discharged home after a week of the therapy.
At 2-week follow-up, the stromal infiltration resolved with small epithelial defect and mild anterior chamber reaction (Fig. 1C). Topical PHMB was tapered down to every 4 hr. At 3-month follow-up, the infiltration became central scar with corneal pannus (Fig. 1D). The pin hole-corrected VA was improved to 6/18 in the affected eye.
DISCUSSION
Unlike systemic Acanthamoeba infection that mostly associated with immunocompromised hosts, AK was generally reported from immunocompetent hosts, especially in CL wearer. The improper usage of CL is believed to be a primarily risk factor of AK outbreak in developed countries such as USA [12], England [13], Hong Kong [14], and Singapore [15]. However, incidence of AK in developing countries is often related with outdoor activities and risk exposures to dust, soil or water [1]. In Thailand, Acanthamoeba have been isolated from clinical and environmental samples [5,16–19]. Despite its ubiquity in the nature, less than 10 AK cases have been previously reported from Thailand [20], bringing up to the curiosity that the true AK incidence may be underestimated.
This study appears to be the first AK case in Thailand occurred in a HIV-infected patient without history of CL wearing. The patient presented atypical ocular findings and was initially misdiagnosed as fungal keratitis. Two months after the onset, delayed diagnosis caused permanent corneal scar and visual loss. The longer diagnostic period and the poorer visual outcome have also been reported in non-CL-related AK series, which are the significant points differed from CL-related AK [2]. In immunosuppressed patients, Acanthamoeba can cause granulomatous encephalitis and disseminated acanthamoebiasis [21]. As an opportunistic role, Acanthamoeba can cause spontaneous keratitis in HIV-infected patients [22] or even polymicrobial infection [23]. The risk of Acanthamoeba keratitis in immunocompromised host has neither been increased than normal host nor associated with a systemically immunocompromised state [24]. The lack of typical signs of Acanthamoeba keratitis, particularly in non-contact lens wearer, can lead to the delay in diagnosis and unsatisfactory outcomes or even loss of the eye [22]. However, the relationship between HIV infection and Acanthamoeba keratitis remained unknown.
For diagnosis, the causative agent in this study was eventually approved by CFW staining, a non-specific fluorochrome extensively used in clinical mycology. Principally, CFW is composed of compounds which has the binding ability to chitin and cellulose on the microorganism’s structures [25]. In parasitological field, CFW is known as an optical fluorescence brightener widely used in the rapid detection for spores of microsporidia [26], cysts of Entamoeba [27], cysts and trophozoites of Giardia lamblia [28], cysts of Pneumocystis carinii [29], eggs of Ascaris lumbricoides [30], larvae of Dirofilaria immitis [31] and cysts of Acanthamoeba [32]. In this study, Acanthamoeba in the specimen appeared mainly the cyst form due to the prolonged infection without therapeutic responses. The endocysts features which made up mainly of cellulose might enhance the detection possibility by CFW. The bright bluish-white fluorescence furnished the endocysts so that they could be easily distinguished from surrounding corneal tissue background. Our results were concordant with the previous investigation and insisted the robustness of CFW staining in AK diagnosis that CFW staining of repeated corneal scrapings was helpful, reliable enough to be the decisive method, especially in non-specific symptomatic cases [33].
The routine tests for AK diagnosis in most laboratories are generally microscopy of corneal scraping and culture. By comparison with smear staining, culture method may take longer detection time which may be up to 2 weeks in some cases depending on quantity of amoebae available in the samples, and the quality of corneal scrapings. Fortunately, in our case, amoebae amount in the scraping was rich enough to produce the clear zone on the agar culture within the second day of incubation. In some cases that microscopic exam showed negative, the culture which simultaneously performed may also be helpful [34]. Moreover, the clinical isolated amoebae harvested from the culture can be used for further epidemiological or genetic studies.
For molecular diagnosis, Acanthamoeba spp.-specific PCR (ACA-PCR) has been recently developed revealing high sensitivity and specificity in the rapid AK detection [7,35]. It was noted from an AK study in non-CL Indian wearer that sensitivity of the PCR would have been higher if the samples were from initial corneal scraping rather than the latter [34]. As Acanthamoeba-specific amplimer S1 (so called ASA.S1) retrieved from the ACA-PCR shows high variability among individual genotypes, the PCR has been extensively used for both clinical and epidemiological objectives. Based on the variations in 18S rDNA sequences, genus Acanthamoeba can currently be classified into 20 diverse genotypes (T1–T20) [11]. Among them, T4 is a major genotype commonly isolated from patients and nature. In the present study, the causative agent CMU_AK was designated as typical T4 genotype which was concordantly affirmed by BLAST and phylogenetic analysis. The sequence of CMU_AK was identical to that of all Acanthamoeba T4 references from keratitis patients and environments but showed 0.73% nucleotide differences from that of the positive control, MU_AK. The clustering of the MU_AK into T4 monophyletic clade, could be explained that the 0.73% of genetic distances were remained lower than the 5% cut off value which is known to be characteristic of different genotype [11]. To our knowledge, few molecular studies of Acanthamoeba isolated in Thailand have been documented [18,19,36,37]. Clinically, genotypes T4 and T10 were reported to be responsible for Acanthamoeba keratitis cases, while only T4 was identified from a case of Acanthamoeba meningoencephalitis [18]. Environmentally, genotypes T3, T4, T5, T9, and T17 were isolated from natural water resources of Thailand [18,19]. It is likely that T4 is the most widespread distribution genotype in the country. Although not all T4 isolates have been reported to possess pathogenic properties, majority of Acanthamoeba infections have been associated with genotype T4 [21]. Therefore, potential pathogenicity of T4 reporting in the country should not be ignored. The disease monitoring in human groups involving the risk activities like agriculture or joining of water-related festivals such as Loi Krathong and Songkran, should be aware.
In conclusions, the clinical manifestations of Acanthamoeba keratitis in HIV-infected patient may be atypical making the diagnosis is delayed and resulting in poor visual outcome. Comprehensive history taking and combination of diagnostic modalities are important to prevent the misdiagnosis. Although Acanthamoeba can play an opportunistic role in HIV-infected patient with suboptimal immune status, it is still unclear whether this influenced the course of the disease.