INTRODUCTION
Myiasis is an infestation of humans and other vertebrates by various dipteran larvae that feed on the host’s dead or living tissues, body secretions, or ingested food [1,2]. Human myiasis is more common in poor socioeconomic regions of tropical and subtropical countries, whereas in industrialized countries of temperate climates it is usually associated with travel or precarious living conditions [1,3]. According to the classification of myiasis in relation to the location on the host, the infestation is classified into sanguinivorous or bloodsucking myiasis, cutaneous, furuncular, and migratory myiasis, wound myiasis, and cavitary myiasis [1]. The most important family of flies causing wound myiasis is Calliphoridae, with Calliphora, Lucilia, Chrysomyia, and Cochliomyia as the most causative genera. Lucilia spp. are present in South Africa, Australia, Europe, and North America. Their larvae prefer to feed on dead tissue, usually causing facultative open wound myiasis [1,4]. Exposed lesions attract and stimulate females to oviposit [1]. Because flies and their larvae are hosts to bacteria, there are possibilities of secondary bacterial infections of the lesions and bacteremia if the bacteria invade the bloodstream. The bacteria most often associated with myiasis-induced bacteremia are Wohlfahrtiimonas spp. and Ignatzschineria spp. [5]. Both genera are well known inhabitants of the larvae of many fly species [6]. In the salivary glands of Lucilia spp. bacteria of the genus Ignatzschineria have been found in relatively high abundance [7]. Here we describe a case of myiasis with subsequent Ignatzschineria larvae circulatory infection, presumably as a complication of a Lucilia sp. maggot wound infestation in a young male migrant.
CASE DESCRIPTION
The ethic review was exempted by the Institutional Review Board of the Institute of Microbiology and Immunology (IMI), Faculty of Medicine, University of Ljubljana, Slovenia (IRB No. 469-TJI/20). All procedures followed were in accordance with the ethical standards of the institutional and national committee on human experimentation and with the Helsinki Declaration as revised in 2013. The patient samples were collected for diagnostic purposes as a part of a routine diagnostic procedure of the IMI. The patient was informed about all diagnostic procedures, and steps were taken to preserve patient anonymity. All samples were anonymized and linked only to randomized numerical codes. Since no additional samples or data were collected, the study was deemed to be low risk and the need for additional ethical permission from the National Medical Ethics Committee was waived.
In September 2019, an 18-year-old male patient, an irregular migrant from Asia, was admitted to the Department of Infectious Diseases, University Medical Centre Ljubljana, Slovenia, after being detained while crossing the border river between Slovenia and Croatia. He had left his home country approximately 2 years before this incident and was travelling on foot in rural areas and woodlands of the Balkans. After falling off a rock 3 days prior to admission, he reported subsequent abdominal pain and pain in the left leg. He had not eaten or had a bowel movement for several days; he had vomited once before admission but passed urine normally. He had no underlying comorbidities, but he reported an allergy due to which he was taking an antihistamine. Physical examination revealed that he was in pain, febrile (39.6°C), and normotensive (blood pressure 114/65 mm Hg) with tachycardia (130 beats/min), and tenderness in the right lower quadrant of the abdomen. There were multiple superficial wounds on both the calves and feet with surrounding cellulitis, which had spread proximally to the right thigh, and tenderness and swelling of the left knee. No accompanying lymphadenopathy was visible. The middle finger of his left hand was also swollen and tender. Numerous small moving white larvae were observed on the skin of the legs, in both armpits, in the perigenital region, and in the skin folds. The wounds were debrided, and all the visible larvae were removed. Urinary retention was noted, and a urinary catheter inserted. Laboratory results revealed an elevated C-reactive protein (CRP) level (499 mg/L), a procalcitonin (PCT) level of 8.94 ng/ml, and leukocytosis of 25.9× 109/L with a left shift (8% bands and 3% metamyelocytes). Acute renal insufficiency was present (plasma creatinine of 168 μmol/L, upper limit 97 μmol/L; and urea of 24.2 mmol/L, upper limit of 7.5 mmol/L). The patient had mild hyponatremia (130 mmol/L, lower limit 135 mmol/L), increased myoglobin of 553.4 μg/L (upper limit 110 μg/L), mild elevations of transaminases (AST 2.63 μkat/L, upper limit 0.52 μkat/L; S-ALT 1.38 μkat/L upper limit 0.57 μkat/L), and increased D-dimer (4,683 μg/L, upper limit 500 μg/L). A soft tissue ultrasound of the right thigh showed cellulitis. Blood cultures were taken, and the patient was started on empirical intravenous antibiotic therapy with flucloxacillin 2 g every 6 hr and ciprofloxacin 400 mg every 12 hr. Following treatment, the patient was afebrile the next day. The patient continued to receive supportive therapy with antipyretics, analgesics, and parenteral hydration, upon which the kidney function normalized within 4 days. A blood smear for malaria was negative. In accordance with our institutional guidelines, surveillance samples were taken on admission. A rectal swab yielded ESBL-producing Enterobacter cloacae, and the pooled skin swab samples grew methicillin-resistant Staphylococcus aureus. Samples for other multidrug resistant organisms were negative.
The wounds were redressed 2 days later, with numerous larvae observed on the calf wounds, in the blisters surrounding the calf wounds, and under the toenails of several toes. All visible larvae were removed and additional debridement of dead skin surrounding the wounds was performed. Where the larvae were rooted in the nailbed, the overlying nails were ablated and the larvae extracted. The area was cleaned with a 3% solution of hydrogen peroxide and dressed. The patient became febrile again within hours after the second debridement, and therefore 2 blood culture sets, each containing one aerobic (BD BACTEC Plus Aerobic/F, Becton Dickinson, Franklin Lakes, New Jersey, USA) and one anaerobic (BD BACTEC Lytic Anaerobic/F, Becton Dickinson) bottle were drawn again and, due to an increase in leukocytosis to 33.1×109/L, antibiotic therapy was changed to tigecycline and vancomycin. The patient was hypotensive the next day (blood pressure 88/55 mm Hg) accompanied by vomiting. We changed tigecycline to imipenem/cilastatin 500 mg every 6 hr i.v. but continued with vancomycin 1 g every 12 hr i.v. Hypotension resolved after a fluid bolus. Acute bowel obstruction was excluded by abdominal radiograph and severe obstipation diagnosed, which was resolved by enema.
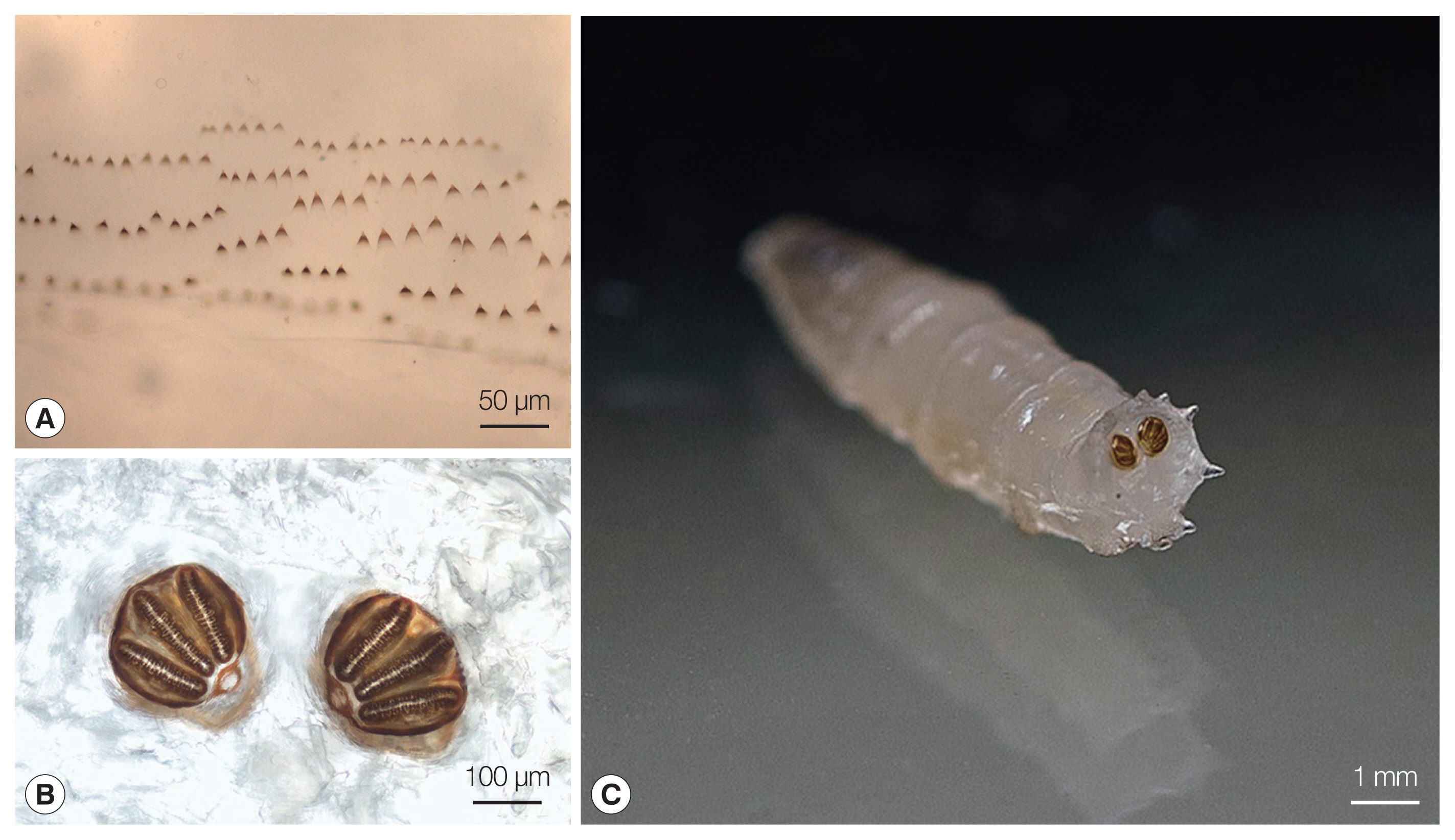
The larvae were sent to the IMI at the Faculty of Medicine in Ljubljana, Slovenia, for identification, where they were preserved in 10% formalin solution. The genus Lucilia was confirmed macroscopically and microscopically at the IMI through their morphological characteristics using the identification key to fly larvae [8]. The larvae were 9 to 10 mm long, round in cross section, tapered anteriorly, and white to cream colored. All of them were in larval stage III (3rd instar) of their life cycle. In the mouthparts an accessory oral sclerite was absent. The dorsal arm of the cephaloskeleton was longer than the ventral arm. The bodies of the larvae had short spines (Fig. 1A). The posterior spiracles were located on the face of the terminal segment, sessile with 3 distinct straight slits surrounded by a complete closed peritremal ring with one internal projection between the outer and middle slits and a distinct button (Fig. 1B, C). The spiracular area was surrounded by 12 tubercles.
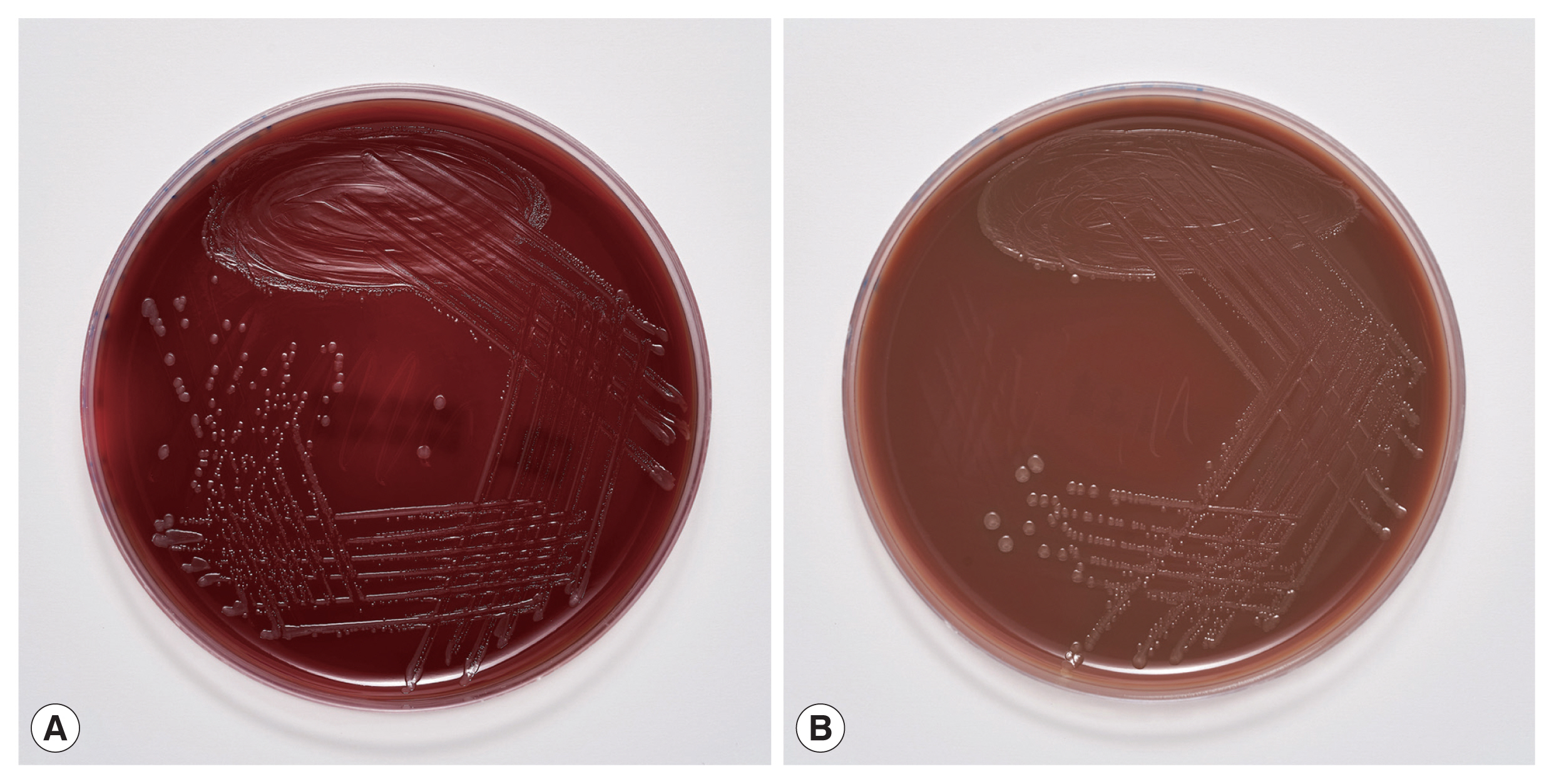
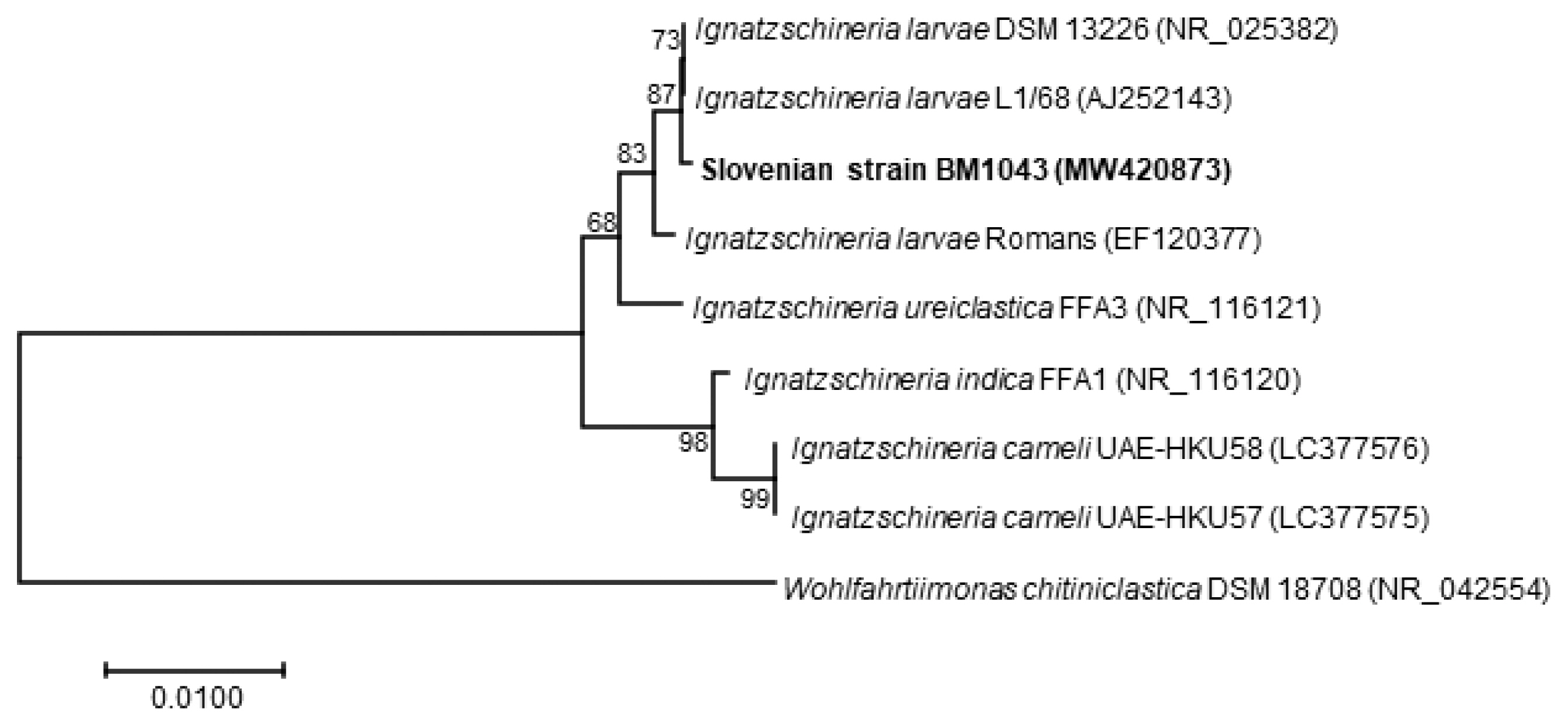
The blood cultures drawn on admission were sterile. However, of the 2 blood culture sets drawn after the second wound debridement, one anaerobic bottle was reported positive by the BD BACTEC FX blood culture system (Becton Dickinson), and Gram staining revealed Gram-negative rods (Fig. 2). Identification of bacteria directly from positive blood culture using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Server version 4.1.70 (PYTH) 48 2016-10-26_15-05-35, Billerica, Massachusetts, USA) [9] was not successful. Subculturing resulted in the growth of colonies with a yellowish pigment on Columbia and chocolate agar (Fig. 3) after 18 hr of incubation. No growth was observed on anaerobically incubated growth media. The identification of bacteria by MALDI-TOF MS was unsuccessful also from subculture. The strain was identified as Ignatzschineria sp. by partial (320 bp long region) 16S ribosomal ribonucleic acid (16S rRNA) gene sequencing [10]. By sequencing a longer 1,462 bp region of the 16S rRNA gene [11], the strain was identified as Ignatzschineria larvae with an identity match of 99.9%. The nucleotide sequence was deposited in the GenBank database under the accession number MW420873. The identity match with the most closely related species was 99.2% for Ignatzschineria ureiclastica and 98.5% for Ignatzschineria indica (Fig. 4). Testing of antibiotic susceptibility was done using Etests (Liofilchem, Roseto degli Abruzzi, Italy or bioMerieux, Marcy-l’Étoile, France). The isolated strain of I. larvae was reported susceptible to piperacillin-tazobactam, ceftazidime, ciprofloxacin, and imipenem considering pharmacodynamic/pharmacokinetic (non-species related) European Committee on Antimicrobial Susceptibility Testing (EUCAST) Clinical breakpoints [12].
After the second debridement, no more larvae were observed in the wounds, which were redressed regularly and were healing well. A swelling was observed on the proximal right thigh, and therefore an abscess was suspected and ultrasound performed, which showed only lymphadenopathy. Because tularemia is present in the rural Western Balkans [13], a punctate of the lymph nodes was sent to the IMI for PCR for Francisella tularensis, which was negative. Serology for Bartonella spp., Francisella tularensis, and Toxoplasma gondii were also negative. Thus, lymph node enlargement was likely only due to skin and soft tissue infections.
After receiving 10 days of appropriate antibiotic therapy, the patient’s clinical condition improved and biomarkers of inflammation resolved. He was afebrile from the 5th day onward and was discharged to a detention center. He was then lost to follow-up.
DISCUSSION
This is the first reported human case of Ignatzschineria spp. bacteremia in Slovenia. Ignatzschineria spp. are emerging aerobic, Gram-negative, non-sporulating, non-haemolytic, non-motile, rod-shaped bacteria that were first isolated from larvae of Wohlfahrtia magnifica, a parasitic flesh fly causing myiasis in several animal species and occasionally in humans [14–17]. Recent reports suggest that Ignatzschineria spp. may also be transmitted by flies from the genus Lucilia, which are more commonly associated with human wound myiasis [5,6]. Bacteria carried by fly larvae can spread into the bloodstream of the infested host, causing serious systemic infections. However, bacteremia in humans caused by species of the genus Ignatzschineria has been rarely described and has only been documented in a few case reports [5,6,15,18–27]. The rarity of infections being detected may be due to the fact that these bacteria were first described only 2 decades ago and also the difficulty of their identification using standard medical microbiology identification methods, such as classical biochemical tests and commercial bacterial identification systems [18]. Recent inclusion of reference spectra for a representative strain of Ignatzschineria indica in the Bruker database may make possible more frequent identification of this species by MALDI-TOF MS in the future [19,28]. Indeed, as reported by Rodriguez-Zuniga et al. [24], Deslandes et al. [19], and Snyder et al. [26], I. indica from blood cultures of patients with sepsis or bacteremia has correctly been identified by MALDI-TOF MS. As shown in our case, the identification of the species I. larvae by MALDI-TOF MS was still not possible.
Currently, the most accurate method for identifying Ignat- zschineria spp. is 16S rRNA gene sequencing. In fact, it was the only way for accurate identification of the species Ignatzschineria larvae in our case. Heddema et al. [21] reported very high similarity among 16S rRNA sequences of I. ureiclastica, I. larvae and I. indica, 3 out of the 4 species described so far in the genus Ignatzschineria. By the initial sequencing of a 320 bp long region of the 16S rRNA gene [10] we were able to identify the bacterium, which caused bacteremia in our patient, at the genus level only. For this reason, sequencing of a 1,462 bp long region of the same gene [11] was required for accurate identification of I. larvae. To date, there have been 4 other case reports describing I. larvae bacteremia in patients with myiasis, all of them originating from France [20,22,25,27]. As in most of the described cases of Ignatzschineria spp. infections associated with myiasis, in these 4 case reports the maggots were discarded after wound cleaning and were not sent for entomological identification. To the best of our knowledge, there is only one case report in which the maggots were identified and Lucilia sericata connected to I. indica bacteremia was determined [6]. The maggots from the wounds of our patient were preserved in formalin immediately upon submission to the IMI. The preservation killed the larvae, made them immotile, and thus made their identification possible. Lucilia sp. was identified based on the maggots’ morphological appearance. Unfortunately, the preservation prevented their subsequent bacterial analysis. Hence, it was impossible to confirm that I. larvae causing bacteremia in our patient formed part of the flora of these maggots. However, a relation between Ignatzschineria spp. infection and maggot infestation is well documented [5,6,25]. Thus, our hypothesis that the bacteremia originated from maggots infesting the patient’s wounds is reasonable. Moreover, the bacterium grew only in the second set of blood cultures, which were drawn after wound debridement that involved nail ablation and necrectomy. We believe it is likely that the wound manipulation caused invasion of the bacteria into the bloodstream and a second spike of fever despite concomitant antibiotic therapy.
As discussed above, Lucilia spp. maggots are among the most common maggots infesting human wounds [6]. Community-acquired myiasis by these larvae poses a risk for serious systemic bacterial infections [5,18]. On the other hand, sterile medical-grade larvae of Lucilia species are very effective at wound cleaning and are therefore used in one of the oldest techniques in wound care, known as maggot debridement therapy or biosurgery [29]. Biosurgery has been acceleratingly reintroduced in healing of chronically infected wounds since the appearance of bacterial strains with antibiotic resistance [30].
Our patient was an irregular migrant from Asia using the western Balkan migration route to reach western Europe. This route is one of the main migration routes into Europe. In 2019, around 14,000 illegal crossings were identified on the European Union’s borders on the western Balkan route alone, more than double the 2018 statistics [31]. In Slovenia, a total of 16,099 illegal crossings of the border were recorded in 2019. The number increased by 73.8% compared to 2018 [32]. Given the increasing number of illegal border crossings, myiasis and myiasis-induced bacteremia could become an important migrant health issue.
Clinicians should have a high level of suspicion when treating patients with neglected wounds. Just as myiasis may indicate an underlying bacterial infection, the presence of bacteremia with Ignatzschineria spp. or Wohlfahrtiimonas spp. –another maggot-associated bacteria that have caused episodes of bacteremia–may indicate myiasis [5]. We would like to emphasize that standard biochemical tests and automated identification systems do not always identify these emerging pathogens. We therefore believe that cases like ours need to be reported.