INTRODUCTION
Toxoplasma gondii, the causative agent of toxoplasmosis, is a parasite capable of evading both phagocytic and non-phagocytic cells of warm-blooded vertebrates, and approximately a third of the world’s population are estimated to be affected by these pathogens. Their infection can have fatal consequences in pregnant women or immunocompromised individuals [1]. Therefore, T. gondii is a pathogenic protozoan organism of global importance [1]. T. gondii infections can be subdivided into acute and chronic stages, which are characterized by the rapid growth of tachyzoites and slow-growing cyst formations predominantly in the brain and musculature. There are 3 distinct clonal lineages of T. gondii and these are classified as type I (RH strain), type II (ME49), or type III based on the parasite’s virulence. While type II and III strains are relatively avirulent and can establish chronic infections, type I strains are highly virulent and uniformly lethal in mice [2,3]. To successfully treat and manage toxoplasmosis patients, as well as estimating disease prevalence, economic loss avoidance, food safety risk evaluation, and establishing epidemic prevention policies at a national level, developing an effective T. gondii diagnostic method for both humans and animals is necessary [4].
Although various direct detection methods are available, such as bioassay, microscopy, or molecular-based assays, these methods are reported to be time-consuming, costly, and possess limited sensitivity. By contrast, multiple serologic tests including indirect haemagglutination test (IHAT), modified agglutination test (MAT), latex agglutination test (LAT), indirect fluorescent antibody test (IFAT), and enzyme-linked immunosorbent assay (ELISA) are more optimized and practical. ELISA, in particular, is widely utilized for its convenience in antigen-specific antibody response detection across various laboratories [5,6]. However, test performance depends mostly on diagnostic antigens. While most conventional tests used today are based on the T. gondii tachyzoite antigens, standardizing this method is difficult due to the presence of non-parasitic components from eukaryotic host cells. Consequently, numerous studies have reported the acquisition of false-positive results along with poor specificity and sensitivity for T. gondii for this method [7]. In the past decade, multiple recombinant antigens with serodiagnostic potential were identified, which includes dense granule proteins (GRA) [8–10], the surface antigens (SAG) [5,11], microneme proteins (MIC), cyst matrix antigen (MAG1), apical membrane antigen (AMA) [12], and the rhoptry proteins (ROP) [13,14]. In the aforementioned studies, recombinant protein antigens were tested using the sera acquired from T. gondii-infected humans and animals. To date, not a single study investigated the relationship between RH or ME49 strain infection dose and the resulting antibody induction in murine models. Rather, in majority of the studies performing recombinant antigen-based ELISA using human T. gondii infection sera, several variables remain unknown such as the duration post-infection, infection dose, and the strain information. Additionally, most studies only assessed IgG response against recombinant protein along with either IgM or IgA. None of them evaluated the responses of all 3 antibody isotypes under identical experimental conditions.
ROP4, belonging to the ROP2 protein family, is expressed at all 3 infective stages of the T. gondii, which includes the tachyzoite, bradyzoite, and the sporozoite. Previously, we have demonstrated that ROP4-expressing VLPs were highly immunogenic and as such, these could be exploited as a potential T. gondii diagnostic marker [15].
In the present study, sera of T. gondii RH and ME49-infected mice were collected and used to evaluate the diagnostic antigen potential of T. gondii ROP4 VLPs by comparing the IgG, IgM, and IgA antibody responses with those of TLA. Findings herein suggest that ROP4 VLP antigen were highly sensitive and enables the detection of T. gondii at an early stage of infection, thus signifying their potential for further development.
MATERIALS AND METHODS
Ethics statement
Seven-week-old female BALB/c mice were purchased from NARA Biotech (Seoul, Korea) and maintained under specific-pathogen-free conditions. All animal experiments were carried out in accordance with the regulations of the Kyung Hee University IACUC (institutional approval number: KHSASP-20-165).
Parasites and cells
Parasites and cells were maintained and harvested as described previously [4]. Briefly, mice were intraperitoneally infected with T. gondii RH strain and ME49 strains. Tachyzoites of RH strain were separated from peritoneal fluids at 5 days PI and resuspended in phosphate-buffered saline (PBS), which were subsequently purified by discontinuous Percoll density gradient centrifugation. ME49 cysts were isolated from the brains of mice at 4 week PI. Spodoptera frugiperda (Sf9) cells were cultured and maintained at 27°C in spinner flasks at 130–135 rpm in serum-free SF900II media (Invitrogen, Carlsbad, California, USA) supplemented with 0.1% penicillin [16].
Generation of recombinant baculoviruses and VLPs
Recombinant baculoviruses (rBVs) and VLPs expressing ROP4 (accession No. EU047558) and M1 (accession No. EF4 67824) were prepared as described previously [15]. Briefly, total RNAs of T. gondii RH strain and influenza A virus (A/PR8/34) were extracted and reverse transcribed for cDNA synthesis. T. gondii ROP4 and influenza M1 genes were amplified by polymerase chain reaction (PCR) and subsequently cloned into the pFastBac vector. Afterward, genes were transformed using DH10Bac competent cells and positive clones were used to generate rBVs. Sf9 cells were co-infected with the M1-rBVs and ROP4-rBVs to generate ROP4 VLPs. Afterward, VLPs were purified through a discontinuous sucrose gradient centrifugation and stored at −80°C until use as described previously [12, 15].
Preparation of tachyzoites lysate antigen (TLA)
TLA was prepared from the harvested T. gondii RH tachyzoites. Briefly, tachyzoites were sonicated and centrifuged at 2,000 rpm for 10 min at 4°C. Supernatant as TLA was carefully collected and protein concentration was determined using the QuantiPro BCA Assay Kit (Sigma-Aldrich, St. Louis, USA) [15,17]. Samples were stored at −20°C until use.
Characterization of VLPs
Toxoplasma gondii ROP4 and influenza M1 proteins were characterized by western blot. After the separated proteins were transferred onto a nitrocellulose membrane, the membranes were blocked and probed with either T. gondii-specific polyclonal antibody or influenza virus anti-M1 monoclonal antibody (Abcam, Cambridge, UK) [19]. Images were acquired using Bio-High voltage EM system (JEM-1400 Plus at 120 kV and JEM-1000BEF at 1,000 kV, JEOL Ltd., Tokyo, Japan) at Korea Basic Science Institute.
Animal experiments
Mice (n=6 per group) were orally infected with 5×103, 1×104, and 5×104 tachyzoites of T. gondii RH strain. For ME49, mice were infected with 10, 50, 150, or 300 cysts through the oral route. For RH-infected mice, blood samples were collected at 1 and 2 week PI. Blood samples of ME49-infected mice were collected at 1,2, 4, and 8 week PI. Blood samples were collected from the retro-orbital plexus puncture and the sera were stored at −20°C until use.
ELISA on antibody production in the mice infected with T. gondii RH or ME49 strain using ROP4 VLPs as antigen
Using TLA or the ROP4 VLPs as antigen, IgG, IgA, and IgM responses were measured by ELISA as described previously [20]. The 96 wells in immunoplates (SPL Life Sciences, Pocheon, Korea) were coated with either TLA or ROP4 VLPs at a concentration of 4 μg/ml in 0.05 M carbonate coating buffer (pH 9.6) and incubated overnight at 4°C. Sera collected from mice were added at 1:100 dilution in PBS into well. After incubating for 1 hr at 37°C, 100 μl/well of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, IgA or IgM (Southern Biotech, Birmingham, Alabama, USA) 1:2,000 in PBS was added. A substrate buffer containing o-phenylenediamine and H2O2 was added and the reaction was stopped by adding H2SO. Absorbance values were measured at 492 nm using EZ Read 400 (Biochrom Ltd., Cambridge, UK) microplate reader.
Statistical Analysis
Data were statistically compared using GraphPad Prism version 5.0 (San Diego, California, USA) through the unpaired Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s post hoc test. In all cases, a difference were considered statistically significant when P-value was smaller than 0.05. All data were presented as mean±SD.
RESULTS
Characters of T. gondii ROP4 VLPs
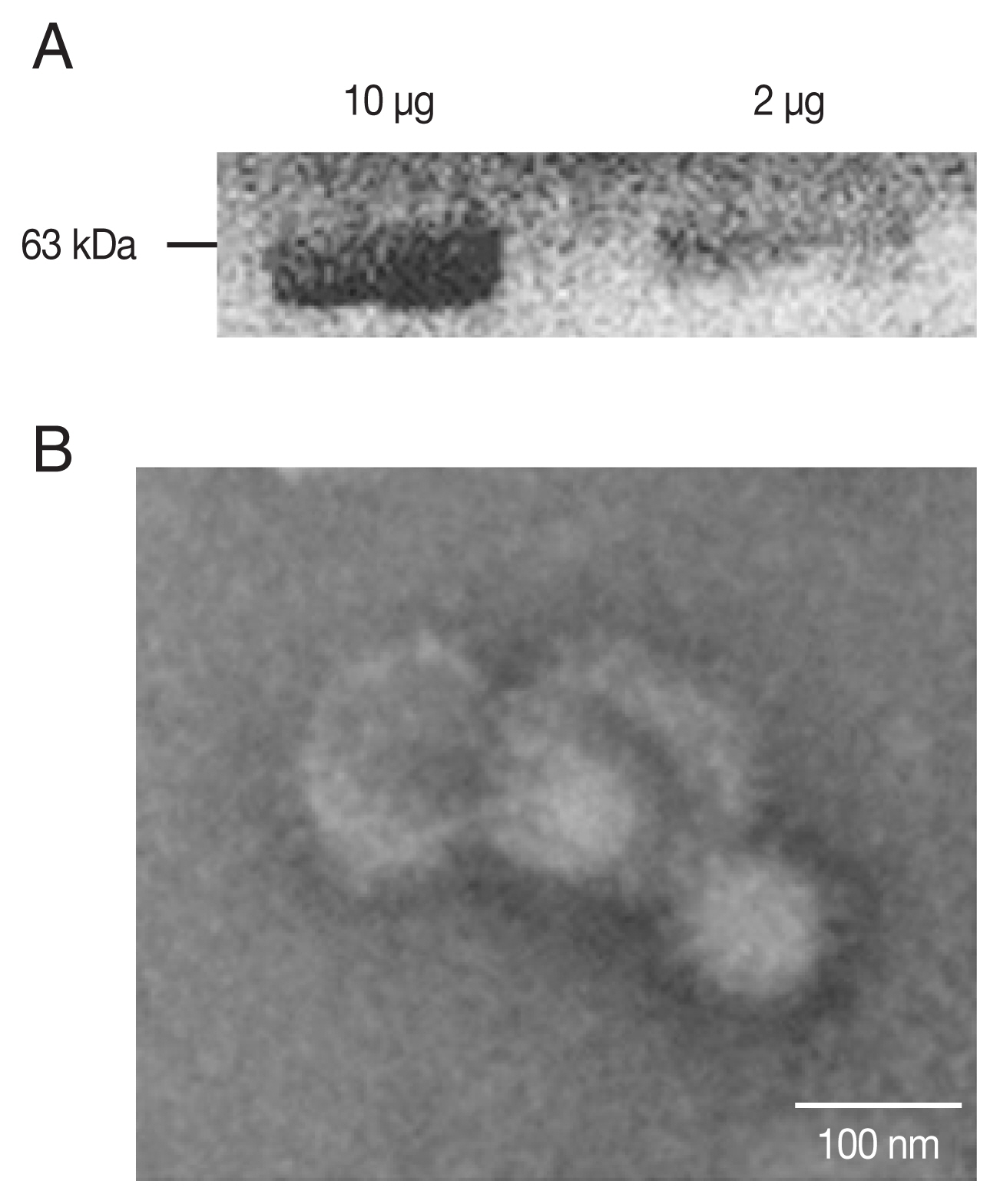
PCR-amplified T. gondii ROP4 gene was cloned into the pFastBac vector as indicated (Supplementary Fig. S1A, B). Baculoviruses expressing the ROP4 were comfirmed (Fig. 1A) by western blot using anti-T. gondii polyclonal antibodies. The spherical morphology of VLPs and the expression of ROP4 antigen spikes on their surface were confirmed by TEM (Fig. 1B).
Sera of T. gondii RH-infected mice strongly interacts with the ROP4 VLP antigen
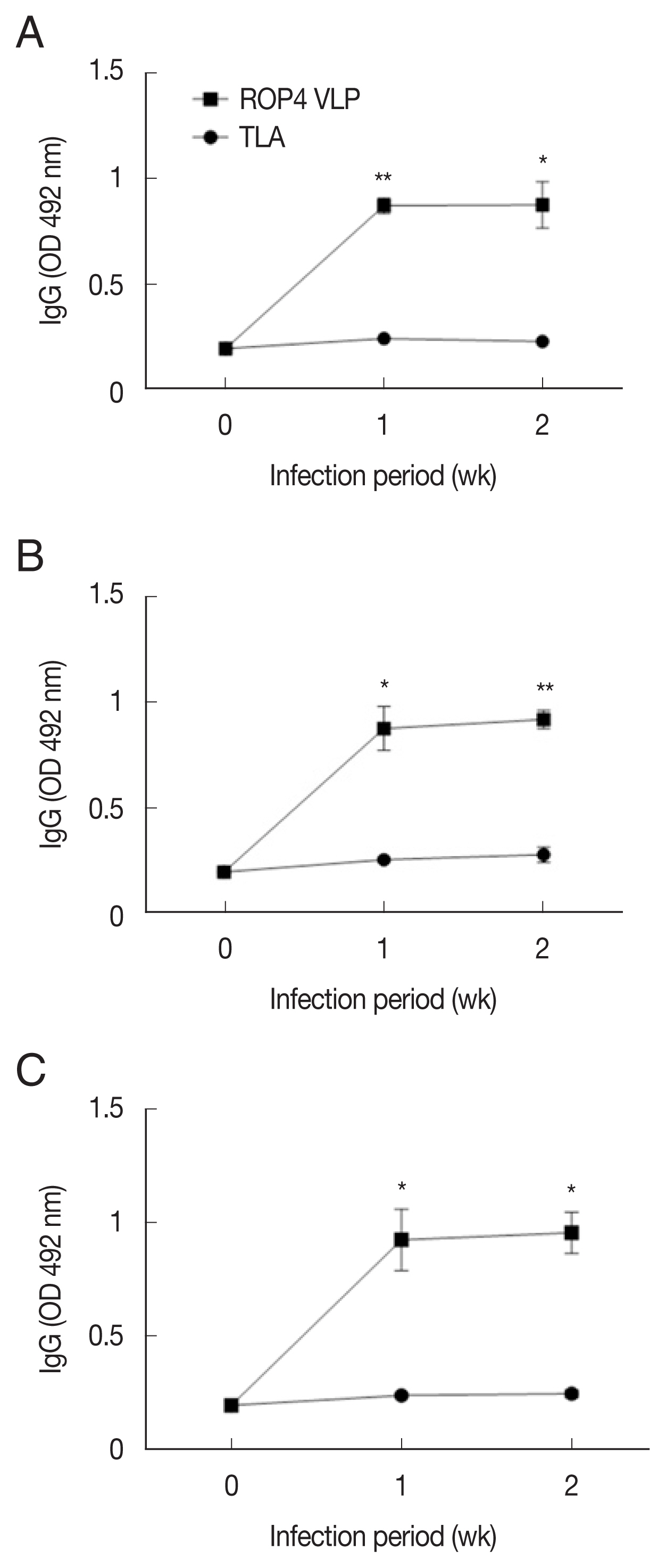
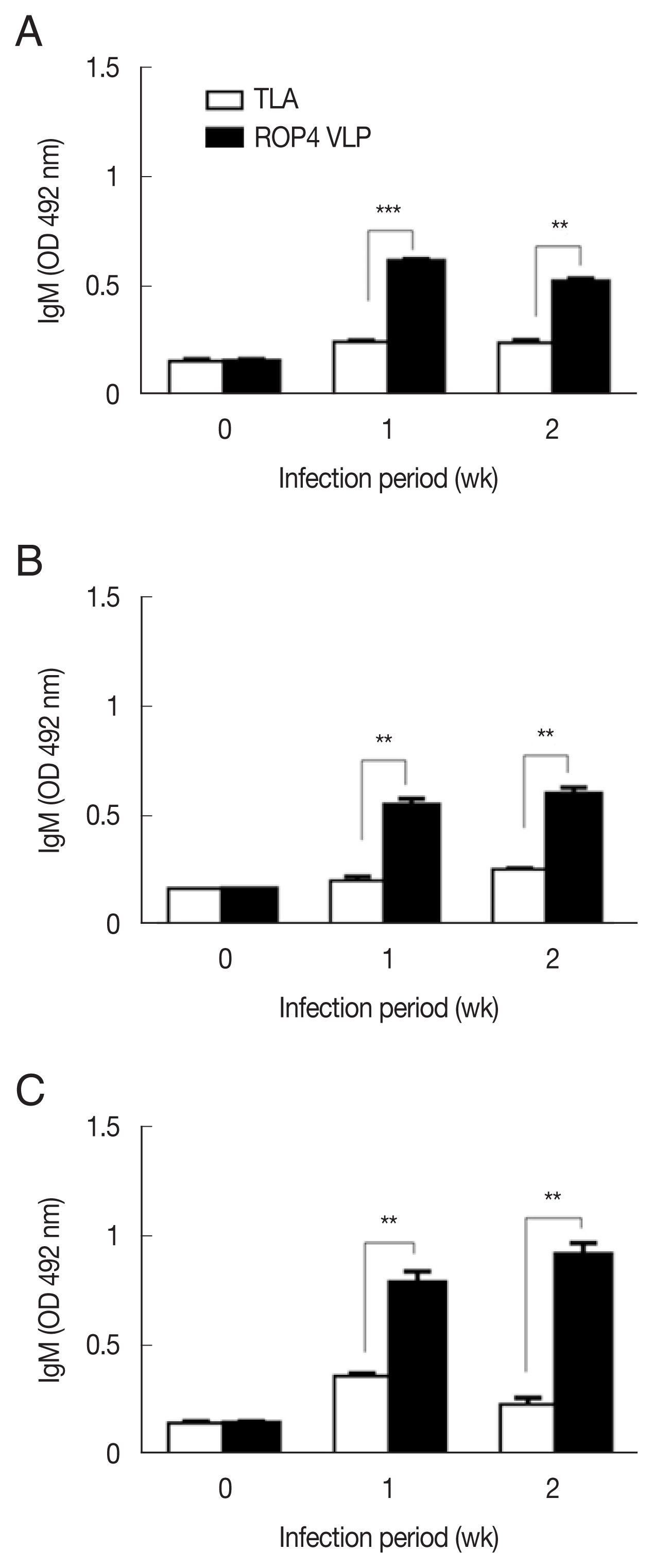
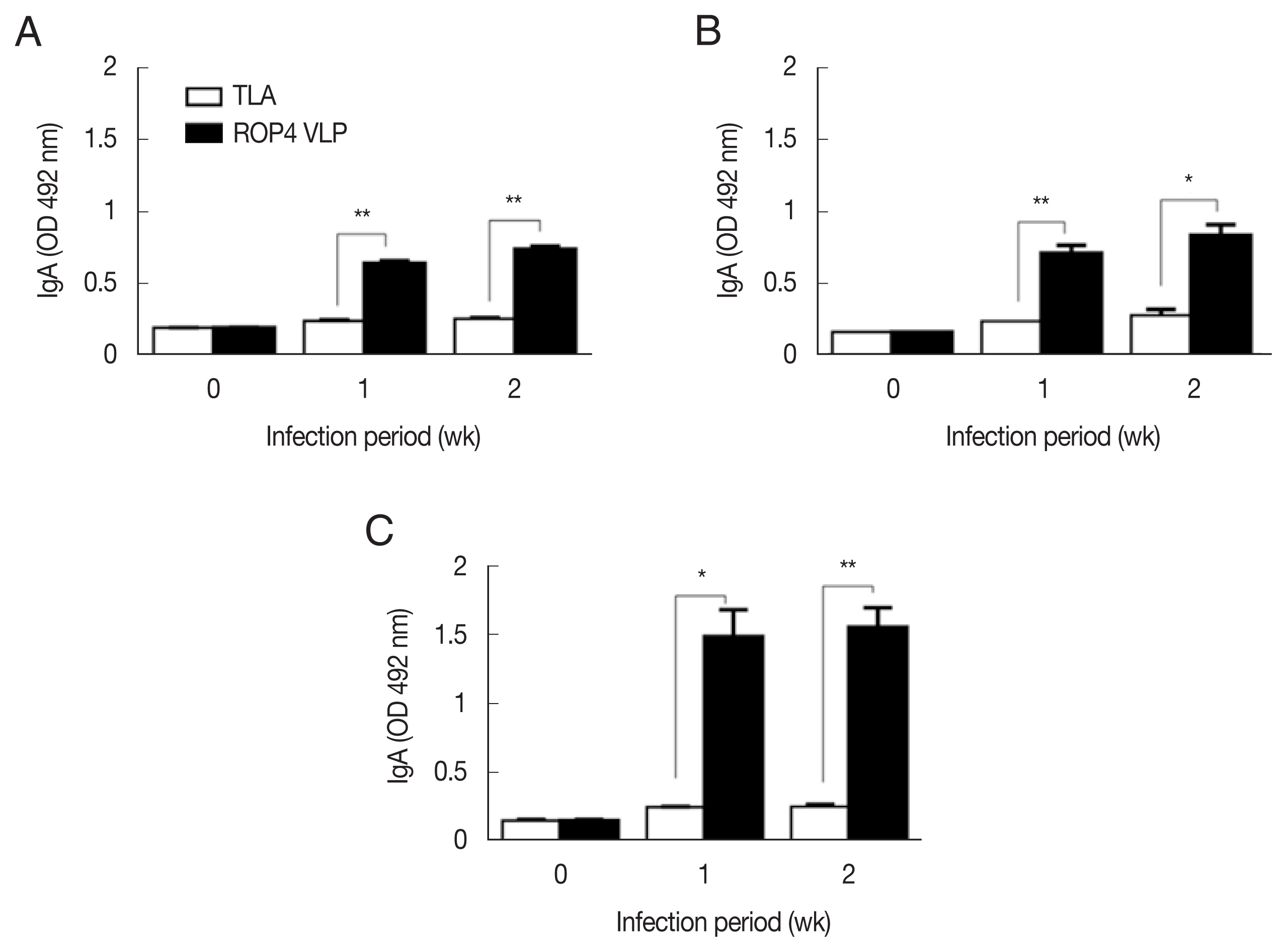
Compared to the TLA antigen, significantly higher IgG responses were detected when sera were reacted with the ROP4 VLPs irrespective of the T. gondii RH infection dose (Fig. 2A–C). The antibody response trends were similar for all infection doses, with a drastic increase in IgG response at 1 week PI, followed by a marginal increase in antibody by 2 week PI. A similar trend was observed in IgM responses. IgM antibody responses appeared in a dose-dependent manner. T. gondii-specific IgM response was much greater in the 5×104 tachyzoite-infected mice sera than those from 5×103 and 1×104 infection groups (Fig. 3A–C). This was the case for IgA response, as indicated by drastically high level of IgA antibody production in 5×104 tachyzoites group (Fig. 4A–C). Importantly, ROP4 VLPs detected IgG, IgM, and IgA antibodies at 1 week PI with T. gondii RH strain, implying that ROP4 VLPs can be employed to diagnose T. gondii RH infection at the early stage.
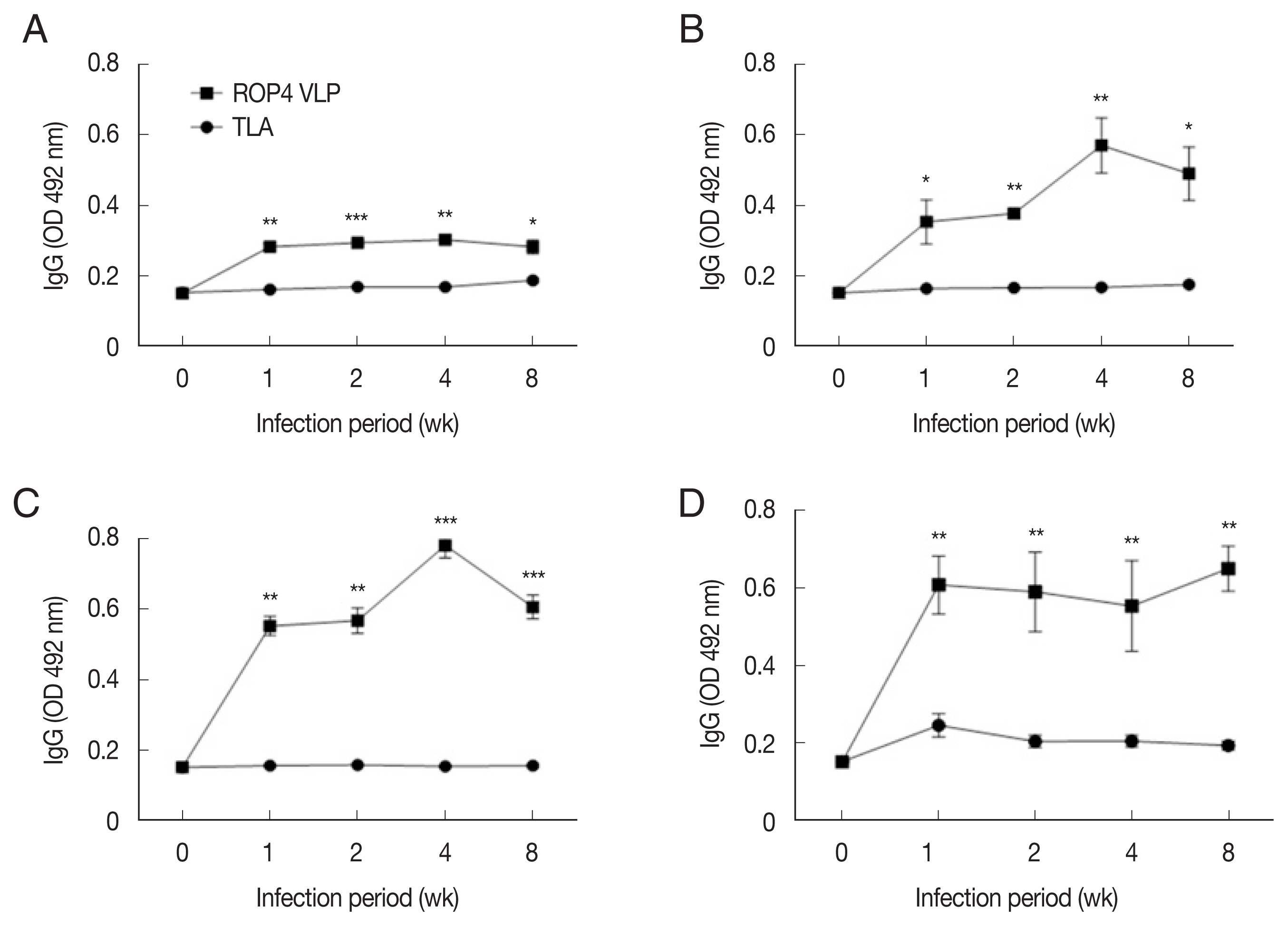
Sera of T. gondii ME49-infected mice strongly reacts with the ROP4 VLP antigens
Compared to the TLA antigen, IgG levels were significantly higher in the ROP4 VLP-coated plates, irrespective of the ME49 infection dose at all time points (Fig. 5A–D). Compared to low infection dose (10 and 50 cysts), antibody production was noticeably high in 150 and 300 cysts groups. Specifically, at 1 week PI, IgG antibody production increased strongly with correlation to infection dose. These results indicate that ROP4 VLP antigen can also be used to detect early stage T. gondii ME49 infection.
DISCUSSION
In the present study, we evaluated the use of VLP antigen for the serodiagnosis of toxoplasmosis induced by T. gondii RH or ME49 infection in mice. Our findings revealed that compared to the conventional TLA antigen, ROP4 VLP antigen can be used as recombinant protein antigens to determine T. gondii RH or ME49 infections with significantly improved sensitivity.
T. gondii RH is highly pathogenic. Consequently, intraperitoneally infected naïve mice usually die within 6 days and renders antibody response detection quite problematic [21,22]. To determine the antibody response induced by T. gondii RH infection, in the present study, mice were orally infected with various doses of T. gondii RH tachyzoites and we found that mice survived over 2 weeks, even when infected with 5×103, 1×104, or 5×104 tachyzoites. This enabled serum collection at 1 and 2 week PI, and the reactivities of ROP4 VLP antigen to the murine sera were detected. We found that ROP4 VLP antign can detect RH infection by significantly increased IgG, IgM, and IgA antibody responses at all measured time points whereas TLA could not, thereby suggesting the use of ROP4 VLP antigen as a recombinant protein in a serologic test of T. gondii RH infection.
Antibody kinetics differ greatly between each antibody isotypes, and this unique pattern seems to be dictated by the time after infections [23]. In general, serum levels of IgM increase during the earliest stage of infection followed by IgA, and the appearance of IgG occurs much later than IgM and IgA [24,25]. Although all of the above antibodies were elicited during the first week upon reaction with the ROP4 VLP antigen, such was not the case for antibody reaction with the TLA antigen at 1 and 2 week PI. ELISA was widely used across various laboratories as a primary diagnostic tool for T. gondii infection for a long time [27–30]. However, it is worth mentioning that both the infection period and dosage of experimental animals in those studies were uncertain. The high sensitivity demonstrated by the TLA ELISA could be resulted from high antibody levels produced with large infection dose, prolonged or even multiple infections. Here, we clarified that in the early stage of RH strain infections, ROP4 VLP antigen might be more suitable than TLA as a diagnostic antigen.
Toxoplasma gondii ME49, unlike the RH strain, results in chronic toxoplasmosis and is relatively avirulent [1,30]. Based on the review of Elsheikha et al. [24], levels of IgG peaks after IgM and IgA at approximately 4 to 8 week PI. In the serodiagnosis of IgM and IgA, we have neither found a representative correlation between infection dosages and response levels nor between time points and that (data not shown). Among studies focusing on detecting IgG responses in mice followed by ME49 cyst infections [31–34], the lowest infection dose was 20 cysts and the earliest time when IgG responses were detected was at 10 days PI. No studies have made experimental animals infected by as few as 10 cysts and no studies successfully detected IgG antibody as early as 1 week PI. Notably, in the present study, mice were infected with cysts of T. gondii ME49 at dosages of 10, 50, 150, and 300 cysts. At all infection doses, ROP4 VLP antigen showed higher IgG antibody responses compared to TLA antigen at 1, 2, 4, and 8 week PI, indicating that antibody induction from even a very low infectious dose can be detected using ROP4 VLP antigen. Importantly, we found that at higher infectious dosage (150 and 300 cysts), ROP4 VLP antigen showed higher IgG antibody responses as early as 1 week PI, which was not reported by using other recombinant proteins [12].
Taken together, this is the first report on application of the T. gondii ROP4 VLP as a coating antigen of ELISA diagnosis in mice. It was found that ROP4 VLP antigen detected T. gondii RH or ME49 infections as early as 1 week PI. Given their sensitivity and convenience, we suggest that ROP4 VLP antigen be a potential candidate for diagnosing both T. gondii RH and ME49 infections.