INTRODUCTION
Sparganosis is caused by a larval tapeworm of Spirometra sp. Human cases have been reported worldwide, but particularly in Asia. Humans can be exposed to sparganosis by consuming undercooked snakes harboring plerocercoids (= spargana), but ingesting infected copepods with procercoids through drinking water is also possible [1]. Although sparganosis may involve internal organs, such as the eye, brain, and spinal cord [2-4], the disease usually manifests with a migrating subcutaneous mass. Since the mass may be non-tender, the larval tapeworm can go unnoticed until it is incidentally discovered during unrelated surgical intervention [5]. In some cases, the precise diagnosis can be missed due to a lack of suspicion by the primary physician. We report here an interesting case of a sparganum infection that is presumed to have lived for 20 years in the leg of a Korean female patient.
CASE REPORT
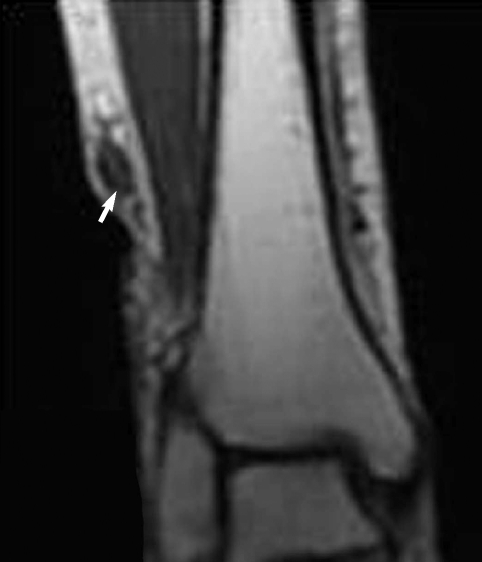
A 60-year-old woman living in Cheonan-si, Chungcheongnam-do, Korea, was admitted to Dankook University Hospital due to pain of the right ankle, aggravated on dorsiflexion. She had noticed a nodular mass at the medial side of the right knee 20 years ago, moving up and down very slowly. She went without any medical care for the lesion, and the mass was not notified for a long time. Three months ago, the mass was found again at the ankle of the patient, developing her ankle pain. On physical examination, an oval, rubber-like mass was found at just proximal to the lateral malleolus of the right foot. The mass was soft, non-tender, and 3 cm in diameter. In addition, another mass was found at the anterior part of the right ankle, and this produced discomfort on dorsiflexion. Routine laboratory tests were unremarkable and no eosinophilia was noted. The plain radiograph of the right leg revealed multiple calcifications extending from the medial part of the knee to the calf (Fig. 1), but nothing was found around the ankle joint. On the MR imaging, heterogeneous signals were observed in the mass, especially near the lateral malleolus (Fig. 2). Epidermoid cyst or dermatomyositis was suspected, and an operation was done on the mass.
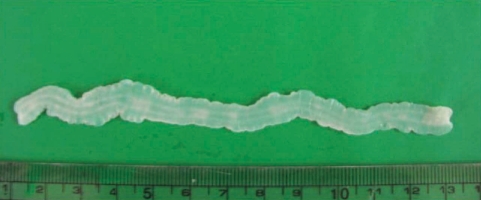
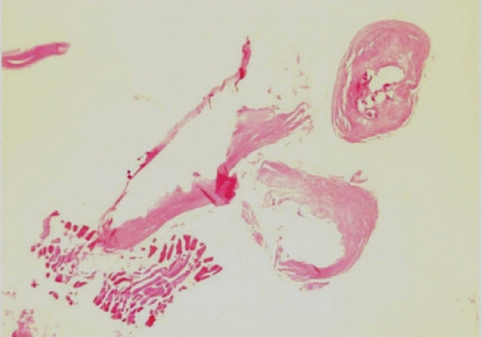
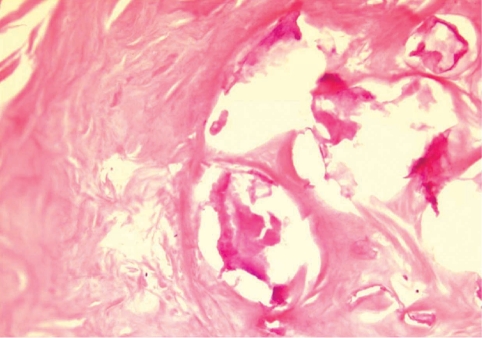
During the surgery, a small longitudinal incision was made on the upper portion of the elongated mass. A white-shiny, synovium-like piece of tissue popped out through the incision, and this was determined to be a sparganum that measured 18 cm in length and 0.5 cm in width, wriggling after removal from the patient. It was diminished to 13 cm in length and 12 mm in width after fixation in 10% formalin (Fig. 3). The calcified foci near the calf were also removed, and the pathologic examination revealed tubular tracts in the subcutaneous tissue (Fig. 4). These tracts possibly represented the pathway along which the larvae had passed. The tracks showed multiple, benign fibrocalcific nodular lesions with a few skeletal muscle fascicles, and the lesions were markedly degenerated (Fig. 5). The patient was a farmer, and she denied ingesting snake or frog meat, but she admitted drinking untreated mineral water from a local mountain. On the third postoperative day, the patient was discharged and followed up uneventfully.
DISCUSSION
Sparganosis has shown a gender preference in Korea, i.e., 94 men and 25 women out of 119 cases [6]. While the men are likely to be infected by eating raw snakes, the source of sparganosis in women may be untreated water containing parasitized copepods, including cyclops. In our case, her habit of drinking water from a local mountain could have led to the sparganum infection. Considering that many people enjoy drinking water in a mineral spring resort, surveys on the mineral water for sparganum infection are needed. Since the route of infection in a Japanese woman was proved to be eating raw carp [7], investigations on fish should also be done.
The longevity of spargana has usually been less than a year based on the characteristics of sparganum causing evident symptoms. However, according to our patient's statement, the present sparganum is presumed to have lived for 20 years in her leg. In our case, the stakeout of the worm during its migration might be responsible for the long-term survival. There have been a few case reports of finding a live worm more than 5 years after the development of a subcutaneous mass. For example, a living worm was recovered from a Japanese woman suffering from a painful nodule for 10 years [7], and 5-year old worm was discovered from a man in the United States [8]. In Korea, a 10-year-survived sparganum was recovered from the patient's thigh [9]. Although the exact time length was not estimated due to the patient's indistinct memory, the sparganum in a Filipino American woman was suspected to have been viable for 19 years [10].
The long-term survival of parasites might be possible due to the ability to modify immune reactions of the host [11]. In sparganosis, it was observed that IgG was cleaved into Fab and Fc fragments by an excreted-secreted (ES) cathepsin-S like protease of Spirometra mansoni plerocercoid [12]. It was also known that the ES products suppress the transcription of inducible nitric oxide synthase, chemokines of KC and JE, tumor necrosis factor (TNF)-α, and IL-1β [13,14]. In addition, a glycoprotein separated from crude ES products of spargana was proved to suppress osteoclastogenesis and proinflammatory cytokine gene expression [15]. Hence, the sparganum might be a valuable model for research alleviating the symptoms of immune-mediated diseases.
In a patient with sparganosis, the characteristic host tissue reaction is an elongated tract-like cavity through which a larva has passed [16]. The sequential pathologic changes of sparganosis were reported as the followings; an inflammatory cell infiltration develops in the first week after the inoculation of the larvae into the soft tissue, tunnel-like structures appeared 2 weeks later, and a fibroblast proliferation appears about 4 weeks after that and this lasts as long as 6 months. If the larva migrates and then dies, the tunnels with inflammatory foci are replaced by granulation tissue [17]. In the present case, the tract was replaced by granulation tissue and calcium nodules were deposited on it. Hence, the tract of this case seems to have been formed at least years ago, supporting an old age of the present worm. However, the comparison of pathologic findings between the present case and other old sparganosis was not possible since the histological examinations of sparganosis were mostly done only on the nodule containing the larva, not the migration tract. For example, histological examination of a 19-year old sparganosis revealed suppurative, granulomatous dermatitis and panniculitis with spaces containing cross-sections of a cestode larval worm [10].
As seen in the Filipino American woman, [10], no peripheral eosinophilia was detected in present case. Hence, if a serpiginous tubular tract is observed during imaging studies, sparganosis should be included in the differential diagnosis despite an absence of peripheral eosinophilia. From the present case, it is speculated that the longevity of spargana could be up to 20 years in man.