AbstractWe report here the records of 10 consecutive Korean patients (10 eyes) with ocular toxoplasmosis which showed the typical clinical manifestations with seropositivity for Toxoplasma gondii specific IgG antibodies by micro-ELISA between 2006 and 2010. Nine patients were males and 1 was female; their age was 50.5±13.8 years. The most common accompanying signs were vitritis (100%), anterior uveitis (70%), and scattered white deposit (80%). Pre-existing retinochoroidal scar was found in 1 (10%) patient. All patients received antiparasitic chemotherapy and systemic corticosteroid treatment, which resolved the presenting attack and recovered the visual acuity better than initial one in 9 patients and worse in 1. Optic atrophy, cataract, and retinal neovascularization were observed during the follow-up period and recurrence was detected in 3 eyes (30%) 6 to 20 months after the initial attack. In Korea, although rarely detected and reported, ocular toxoplasmosis needs more attention in clinical field of retinal diseases.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite which infects both humans and animals as a zoonotic pathogen widespread in nature [1,2]. However, the prevalence of the disease and sources of infection vary among geographic regions with different climates, eating habits, and hygiene status [3-6].
Toxoplasmic retinochoroiditis is the major cause of visual impairment in high T. gondii endemic regions of the United States and the European nations, where it accounts for 30-50% of the posterior uveitis [7]. In Korea, however, the detection of retinal diseases caused by T. gondii is limited [8,9], and the seroprevalence of T. gondii has been reported to be around 2-7% among the general population in the 1980s [10,11].
Ocular involvement can be a result of acquired infection, or more commonly, a recurrence of the congenital form of the disease [12]. More recent reports support the view that acquired infections might be a more important cause of ocular diseases than previously assumed [13-15]. Toxoplasmosis is a progressive and recurrent disease, with which vision-threatening complications, such as retinal detachment, chorioretinal anastomosis, and choroidal neovascularization, may occur any time in the clinical course of the disease. For this reason, patients should be followed periodically to reduce the occurrence of the late complications.
In this study, we report the clinical features, recurrence rate, complications, treatment, and visual results of 10 Korean patients as the first series study of Korean cases with acute symptomatic ocular toxoplasmosis.
CLINICAL FEATURES OF CASESWe retrospectively analyzed the medical records of 10 consecutive patients of 9 males and 1 female diagnosed as active ocular toxoplasmosis at the Uveitis Service of the Ophthalmology Departments of Uijeongbu St. Mary's Hospital and Seoul St. Mary's Hospital from 2006 to 2010 and with at least 6 months follow-up. The demographic and general characteristics of the patients are listed in Table 1. Six of the 10 patients lived at northern areas of Gyeonggi-do or Gangwon-do (province), well-preserved wildlife areas for wild boar and deers near the demilitarized zone (DMZ) facing North Korea. Actually, 5 of 10 patients (50%) had the definite eating history of wild boar meat or deer blood.
The diagnosis of active ocular toxoplasmosis was based on the acute onset of visual symptoms and the presence of characteristic focal retinochoroidal inflammation with or without a hyperpigmented retinochoroidal scar (Fig. 1) combined with serological evidence of Toxoplasma infection.
All of the patients underwent a complete ophthalmologic examination at each visit, including best-corrected Snellen visual acuity, slit-lamp biomicroscopy, tonometry, funduscopy with a Goldmann 3-mirror lens, and indirect ophthalmoscopy of fluorescein angiography. Initial visual acuity was better than 20/40 in 6 (60%) eyes, between 20/200 and 20/40 in 4 (40%) eyes, and less than 20/200 in none. At the initial examination, all patients had unilateral involvement without any scars except for 1 with pre-existing retinochoroidal scars. All active lesions were 2 disc diameters in size or smaller. Ocular findings included retinochoroiditis in 7 patients (70%), papillitis in 1 (10%), and periphlebitis in 4 (40%) eyes (Table 2). Active retinochoroidal lesion was located at the central retina in 2 patients (20%), peripheral retina in 3 (30%), and peripapillary retina in 2 (20%) eyes. All eyes with active lesions showed vitritis, and 7 eyes (70 %) had anterior uveitis. None of these patients had associated risk factors, such as immunosuppression, that might have caused atypical attack. Only 1 eye previously received systemic steroid treatment for posterior uveitis.
Serologic tests were performed by T. gondii-specific IgG antibody micro-ELISA [16], which detected all the sera as positive with OD from 0.26 to 0.78. Complete blood cell counts and erythrocyte sedimentation rate, liver and kidney function test, chest X-ray, and serum angiotensin converting enzyme level were within normal ranges in all patients. Serologic tests for syphilis and human immunodeficiency viral infection appeared as negative.
Our treatment protocol included clindamycin (300 mg, 4 times per day) and trimethoprim-sulfamethoxasol (160 mg and 800 mg each, twice a day). Oral corticosteroid therapy (prednisolone 30-40 mg, once a day) was initiated 1 week after the administration of antiparasitic treatment to check the drug response and always discontinued before antiparasitic treatment was stopped.
After the resolution of the presenting attack, visual acuity became equal or better than the initial in 9 patients (90%) and worse in 1 patient (10%). In the worsened patient, tractional retinal detachment near toxoplasmic scar was slowly progressed during the 2 years of following period, and then the patient underwent vitrectomy and scleral encircling. Anti-toxoplasmic IgG test was performed with vitreous sample in this patient, which resulted in a positive result (Fig. 2). Three (30%) patients had recurrence, of which 2 occurred within 6 months after the first attack and the other at 20 months (Fig. 3). Tractional detachment and a medication-associated complication (i.e., diarrhea) were reported during the follow-up period.
DISCUSSIONTen cases of ocular toxoplasmosis were presented here with the typical clinical manifestations supported by positive serological antibody titers against T. gondii. Ocular toxoplasmosis is one of the most common types (30-50%) of infectious uveitis affecting the posterior pole in countries with high endemicity (30-80% among human population) of T. gondii infection [7]. In Korea, however, there is difference in toxoplasmic environments, such as low prevalence and titers of antibody caused by eating behaviors, pet-loving preferences, and residential changes [17]. Also, there is a significant difference in gender ratio; 9 of 10 patients were males. It may be due partly to the fact that still the social activities of males is more popular than females in Korea and also to a medicinal misbelief that raw viscera of wild animals had beneficial effects for man's stamina.
The fact that only 1 (10%) patient had pre-existing retinochoroidal scars in our study also supports the hypothesis that acquired infections are more frequent causes than congenital infections in ocular toxoplasmosis. Also, the recurrence rate (30%) was lower than that of other overseas studies [2,3,6], although the recurrence found in our study had limitations related with the short follow-up period and small number of cases as well. Though it may be due, in some degree, to differences in life style, such as eating habits and hygiene, it is necessary to find ethnic or genetic differences in standards of lives between Korea and other developed countries.
We chose a treatment strategy of administration of clindamycin and trimethoprim-sulfamethoxasol with oral corticosteroid therapy instead of more effective Fansidar (pyrimethamine-sulfadoxin, Roche). This was because of concerns about Stevens-Johnson syndrome (SJS) sometimes experienced during the treatment of toxoplasmosis, even though no reports relating to T. gondii infection and SJS have been reported so far in Korea.
Recurrence of ocular toxoplasmosis may be obvious when the pre-existing scars are observed. In our report, only 1 of 3 recurred patient showed pre-existing scars, which reflected that the others were newly infected or reactivated in the eye from a chronic infection with brain cysts [9]. A half of the patients resided in northern parts of Gyeonggi-do and Gangwon-do near the DMZ and had experience of consumption of wild boar meat or deer blood, which are assumed to have been from well-preserved nature in those regions. Furthermore, the number of stray cats, and accordingly the oocyst-shedding cats, has been increasing in those regions [18-20] as a risk factor for T. gondii infection.
Therefore, although rarely detected in clinical fields, T. gondii should be considered as an infectious pathogen responsible for the ocular symptoms, such as retinochoroiditis or uveitis. We recommend to check the antibody titers against T. gondii as a routine item for those patients.
REFERENCES1. Klaren VN, Kijlstra A. Toxoplasmosis, an overview with emphasis on ocular involvement. Ocul Immunol Inflamm 2002;10:1-26. PMID: 12461700.
2. Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 2003;136:973-988. PMID: 14644206.
4. Garweg JG. Determinants of immunodiagnostic success in human ocular toxoplasmosis. Parasite Immunol 2005;27:61-68. PMID: 15882232.
5. Tugal-Tutkun I, Corum I, Otuk B, Urgancioglu M. Active ocular toxoplasmosis in Turkish patients: a report on 109 cases. Int Ophthalmol 2005;26:221-228. PMID: 17318320.
7. McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. Am J Ophthalmol 1996;121:35-46. PMID: 8554079.
8. Choi WY, Nam HW, Kwak NH, Huh W, Kim YR, Kang MW, Cho SY, Dubey JP. Foodborne outbreaks of human toxoplasmosis. J Infect Dis 1997;175:1280-1282. PMID: 9129105.
9. Kim MH, Choi YK, Park YK, Nam HW. A toxoplasmic uveitis case of a 60-year-old male in Korea. Korean J Parasitol 2000;38:29-31. PMID: 10743356.
10. Choi WY, Nam HW, Youn JH, Kim WS, Kim WK. Toxoplasma antibody titers by indirect latex agglutination test in patients of Kangnam St. Mary's Hospital and Cheju Medical Center. Korean J Parasitol 1989;27:171-175.
11. Choi WY. Diagnosis and epidemiology of toxoplasmosis in Korea. Korean J Parasitol 1990;28(suppl):41-44.
13. Glasner PD, Silveira C, Kruszon-Moran D, Martins MC, Burnier M Jr, Silveira S, Camargo ME, Nussenblatt RB, Kaslow RA, Belfort R Jr. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol 1992;114:136-144. PMID: 1642287.
14. Holland GN. Ocular toxoplasmosis: new directions for clinical investigation. Ocul Immunol Inflamm 2000;8:1-7. PMID: 10896456.
15. Atmaca LS, Simsek T, Batioglu F. Clinical features and prognosis in ocular toxoplasmosis. Jpn J Ophthalmol 2004;48:386-391. PMID: 15295668.
16. Choi WY, Nam HW, Youn JH, Kim DJ, Kong Y, Kang SY, Cho SY. Detection of antibodies in serum and cerebrospinal fluid to Toxoplasma gondii by indirect latex agglutination test and enzyme-linked immunosorbent assay. Korean J Parasitol 1992;30:83-90.
17. Yang HJ, Jin KN, Park YK, Hong SC, Bae JM, Lee SH, Choi HS, Hwang HS, Chung YB, Lee NS, Nam HW. Seroprevalence of toxoplasmosis in the residents of Cheju island, Korea. Korean J Parasitol 2000;38:91-93. PMID: 10905070.
18. Kim HY, Kim YA, Kang S, Lee HS, Rhie HG, Ahn HJ, Nam HW, Lee SE. Prevalence of Toxoplasma gondii in stray cats of Gyeonggi-do, Korea. Korean J Parasitol 2008;46:199-201. PMID: 18830064.
19. Lee SE, Kim JY, Kim YA, Cho SH, Ahn HJ, Woo HM, Lee WJ, Nam HW. Prevalence of Toxoplasma gondii infection in stray and household cats in regions of Seoul, Korea. Korean J Parasitol 2010;48:267-270. PMID: 20877509.
20. Lee SE, Kim NH, Chae HS, Cho SH, Nam HW, Lee WJ, Kim SH, Lee JH. Prevalence of Toxoplasma gondii infection in feral cats in Seoul, Korea. J Parasitol 2011;97:153-155. PMID: 21348626.
Fig. 1Fundus photographs and fluorescent angiograms of a 50-year-old farmer with active ocular toxoplasmosis on initial presentation (A, B) and after anti-parasitic medications (C, D). 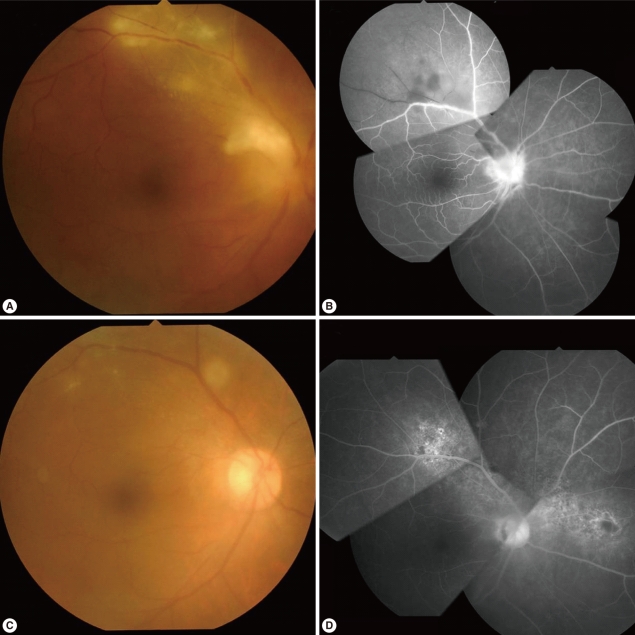
Fig. 2Fundus photographs, B-mode ultrasonography and retinal optical coherence tomography of a 44-year-old man with retinal detachment associated with active ocular toxoplasmosis. A: initial active retinochoriditis and vitritis, B, C, D: fundus photographs, B-mode ultrasonography and retinal optical coherence tomography showing the retinal detachment. E: postoperative mosaic photograph. 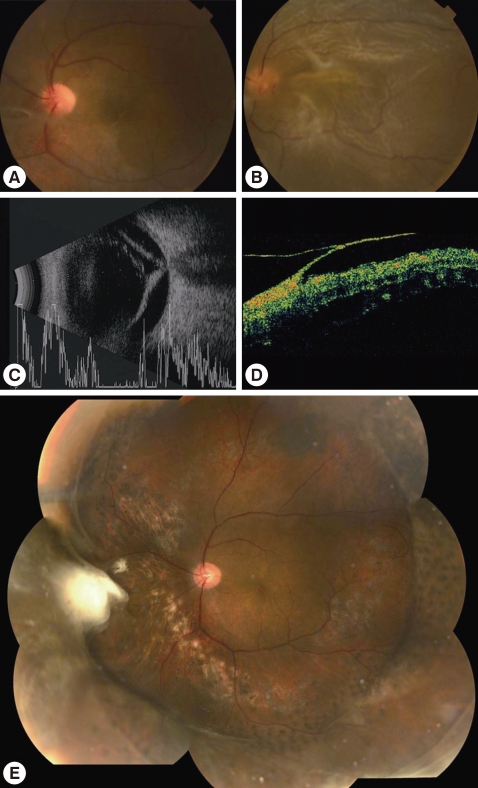
Fig. 3Fundus photographs and fluorescent angiograms of a 27-year-old soldier with recurrent ocular toxoplasmosis on initial presentation (A), first attack finding (B), and second attack finding (C). Note that recurrent lesions were located near the pre-existing scar. 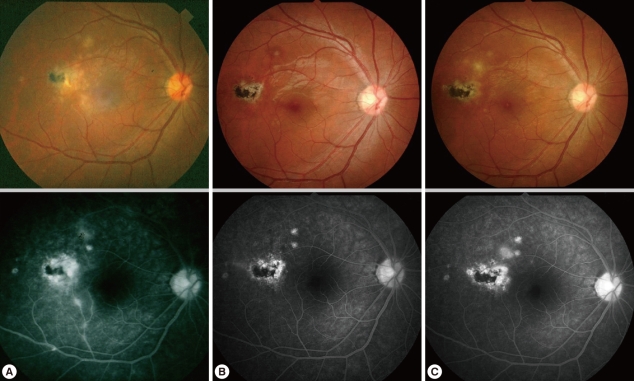
Table 1.Demographic and general characteristics of the patients Table 2.Ocular manifestations and localization of the retinochoroidal lesions
a The area of fundus involvement by active lesion was defined as central retina when the lesions were located within the temporal vascular arcades but not within 1 disc diameter from the optic disc, or as peripapillary within 1 disc diameter from the optic disc. Lesions located elsewhere in the retina were defined as peripheral retina. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||