INTRODUCTION
Acanthamoeba is a free-living protozoan pathogen that has been isolated from diverse environments, including air, soil, tap water, swimming pools, and is known to be one of the most ubiquitous protozoans [1-3]. With the wide environmental distribution, they must effectively interact with a range of microorganisms to ensure their survival. It is not surprising that Acanthamoeba have been shown to interact with viruses, bacteria, algae, yeast, and other protists [4-7]. However, it is the ability of Acanthamoeba interacting with host bacterial pathogens that has gained most attention. For example, several studies have shown that Acanthamoeba act as a reservoir for a variety of bacterial pathogens, including Escherichia coli K1 (causative agent of meningitis) [1,8,9], Legionella pneumophila (causative agent of Legionnaire's disease) [10], Coxiella burnetii (causative agent of Q fever) [11], Pseudomonas aeruginosa (causative agent of keratitis) [12], Vibrio cholerae (causative agent of cholera) [13], Helicobacter pylori (causative agent of gastric ulcers) [14], Listeria monocytogenes (causative agent of listeriosis) [15], E. coli O157 (causative agent of hemolytic uremic syndrome) [16], and Mycobacterium avium (causative agent of respiratory infections) [17] and may act as vectors to transmit these pathogens to susceptible hosts.
The endosymbiotic relationship of bacteria with amoeba may serve (i) to protect bacteria in hostile environments, (ii) the amoebic intracellular environment might assist bacteria to adapt to survival in mammalian phagocytic cells, suggesting that amoeba-bacteria are involved in complex interactions. Further complexity is attributed to the fact that bacteria are a favourable food source for Acanthamoeba. What determines amoeba to act as a host for bacteria and at other times to consume bacteria remains incompletely understood. The fate of bacteria inside Acanthamoeba may be most likely dependent on the virulence properties of bacteria and/or Acanthamoeba. Based on this, it is hypothesized that the ability of bacteria to resist amoebic killing may have led to their evolution to produce human diseases, i.e., evade human immune cells, such as phagocytes. Therefore, amoebae may be the missing link to bacteria that are distributed in the environment and those causing human infections.
Most bacteria contain some sort of a polysaccharide layer outside the cell wall or outer membrane. In a general sense, this layer is called a capsule. Capsules are known to protect bacteria from engulfment by predatory phagocytes and from attack by antimicrobial agents. As related with previous studies, invasive E. coli K1 interacted with A. castellanii trophozoites more than non-invasive E. coli K12, which meant more association, invasion, and survival of E. coli K1 with A. castellanii [1,8]. In particular, a capsule-deletion mutant of E. coli K1 (lacking the neuDB genes cluster that is necessary for the production of cytoplasmic precursors to the exopolysaccharide capsule) exhibited significantly reduced association compared with the wild type strain and limited ability for invasion/uptake by and survival inside Acanthamoeba. The bacterial capsule would be a very important factor for the interactions with amoeba. However, the importance of a somatic antigen as other virulence factor in E. coli is not clearly understood. E. coli K5 is a streptomycin-resistant strain of the serotype O7:K1 [18]. E. coli K1 and E. coli K5 possess different somatic antigens but an identical capsular antigen.
Here, to understand E. coli-interactions with amoeba using E. coli strains possessing different somatic antigens, we compared and analyzed the E. coli K1 and E. coli K5 interactions with A. castellanii using association, invasion, and intracellular survival assays in A. castellanii trophozoites and cysts.
MATERIALS AND METHODS
Culture of Acanthamoeba trophozoites and E. coli
All chemicals were purchased from Sigma Laboratories (Poole, Dorset, England), unless otherwise stated. A clinical isolate of A. castellanii belonging to T4 genotype, isolated from a keratitis patient (American Type Culture Collection, ATCC 50492) was used. A. castellanii isolate was grown without shaking in 15 ml of PYG medium [proteose peptone 0.75% (w/v), yeast extract 0.75% (w/v), and glucose 1.5% (w/v)] in T-75 tissue culture flasks at 30℃ as previously described [19]. The media were refreshed 17-20 hr prior to experiments. This resulted in more than 95% amoebae in the trophozoite forms, which were subsequently used in experiments. A laboratory non-invasive E. coli strain HB101 (K12) was used as a non-pathogenic bacteria. The E. coli K1 strain E44 used in the present study is a rifampicin-resistant mutant of strain RS218 (serotype O18:K1). This strain is a clinical isolate derived from the cerebrospinal fluid (CSF) of a neonate with meningitis. In particular, E. coli K5 is a streptomycin-resistant strain of serotype O7:K1 and were cultured with streptomycin (25 µg/ml) at 37℃ overnight before use.
E. coli association assay
To study E. coli interactions with live Acanthamoeba, an association assay was performed as was done in a previous report [9]. Briefly, Acanthamoeba were prepared in 24-well plates. The cells were washed once with PBS and incubated with E. coli strains (2×106 cfu/well/0.5 ml of PBS). The plates were incubated for 1 hr at room temperature. Following this incubation, amoebae were washed with PBS for 3 times to remove non-adherent bacteria and amoebae counted using a hemacytometer. Finally, amoebae were lysed by adding SDS (0.5% final conc.) to each well for 30 min, and the number of bacteria was enumerated by plating on nutrient agar plates. The percent bacterial association was calculated as follows: recovered E. coli (cfu)/total E. coli (cfu)×100=% E. coli associated with Acanthamoeba. In addition, the ratio of bacteria to amoebae was calculated as follows: recovered E. coli (cfu)/number of Acanthamoeba=E. coli/Acanthamoeba ratio.
E. coli invasion assay
To determine the ability of bacteria to invade or be taken up by Acanthamoeba, an invasion assay was performed. Briefly, amoebae were grown to confluency in 24-well plates followed by the addition of 2×106 of E. coli as described above. After 1 hr incubation, Acanthamoeba were washed with PBS for 3 times, followed by an addition of gentamicin (100 µg/ml, final concentration, for 45 min) to kill extracellular bacteria. Finally, amoebae were counted, and the intracellular bacteria were enumerated as described above. The percent bacterial invasion/uptake was calculated as follows; recovered E. coli (cfu)/total E. coli (cfu)×100=% intracellular E. coli. In addition, the ratio of bacteria to amoebae was calculated as follows; recovered E. coli (cfu)/number of Acanthamoeba=E. coli/Acanthamoeba ratio.
Intracellular survival assay
To determine the long-term effects of Acanthamoeba and E. coli interactions, an intracellular survival assay was performed. Briefly, amoebae were incubated with E. coli, followed by an addition of gentamicin for 45 min. After this incubation, Acanthamoeba were washed for 3 times with PBS and subsequently incubated in 0.5 ml of PBS for 24 hr at 30℃. Finally, amoebae and E. coli were enumerated as described above and intracellular bacteria calculated as follows: recovered E. coli (cfu)/total E. coli (cfu)×100=% intracellular E. coli after 24 hr in PBS. In addition, the ratio of bacteria to amoebae was calculated as follows: recovered E. coli (cfu)/number of Acanthamoeba=E. coli/Acanthamoeba ratio after 24 hr in PBS.
Encystment assay
An encystment assay was performed as previously described [20]. In the invasion assay, 24-well plates were added with gentamicin and then washed with PBS mentioned above. All mixture from a 24-well plate was transferred into a 1.5 ml eppendorf tube. After the tube was centrifuged, the supernatant was removed, and 50 µl PBS was added into the tube. Then, the 50 µl mixture was inoculated on 3% non-nutrient agar, that is, purified agar (Oxoid, Basingstoke, Hampshire, England) plates. The plates were incubated at 30℃ for up to 48 hr. The encystment of Acanthamoeba was periodically observed under a light microscope.
Bacterial survival from Acanthamoeba cysts induction
After non-nutrient agar surfaces were soaked with 10 ml of PBS by shaking, cysts on the non-nutrient agar plates were gently scraped-off the agar surface using a cell scraper. Cysts were collected by centrifugation at 3,500 rpm for 10 min, washed 3 times with PBS, transferred into a 1.5 ml eppendorf tube with 700 µl of PBS and used in subsequent survival assays. One hundred µl of 700 µl mixture was added into a 24-well plate for a 0 hr survival assay, followed by the addition of SDS (0.5% final concentration) to solubilize any remaining trophozoites as described above. Two 100 µl of 600 µl mixture was added into a 24-well plate containing 900 µl of PYG media for a 43 hr survival assay at 30℃. Two 200 µl of 400 µl mixture was added into a 24-well plate containing PYG and gentamicin (100 µg/ml) for 45 min at 30℃. At the experimental time point, SDS (0.5%) was added to solubilize any remaining trophozoites as described above. Finally, amoebae and E. coli were enumerated as described above and intracellular bacteria calculated as follows: recovered E. coli (cfu)/total E. coli (cfu)×100=% intracellular E. coli after 22 hr and 43 hr in PYG. In addition, the ratio of bacteria to amoebae was calculated as follows: recovered E. coli (cfu)/number of Acanthamoeba=E. coli/Acanthamoeba ratio after 22 hr and 43 hr in PYG.
RESULTS
E. coli K5 exhibited significantly lower association with A. castellanii as compared to the invasive E. coli K1
To determine the ability of E. coli K5 association with A. castellanii, the association assay was performed. Our findings revealed that the invasive E. coli K5 exhibited significantly lower association with A. castellanii as compared to the invasive E. coli K1 for 1 hr (Fig. 1) (P<0.05). Otherwise, the association of E. coli K5 was very similar to the non-invasive E. coli K12. Next, to determine the ratio of E. coli with A. castellanii, amoebae were enumerated prior to bacterial counting. Our results revealed that the ratio of E. coli K1 with A. castellanii was 4.3 cfu per amoeba (0.9 cfu per amoeba for K12) for 1 hr. In particular, the ratio of E. coli K5 with A. castellanii was 1 cfu per amoeba for 1 hr. E. coli K5 exhibited significantly lower association with A. castellanii as compared to E. coli K1, suggesting that the somatic antigen but not a capsular antigen might be an important factor that interacted with A. castellanii trophozoites.
E. coli K5 exhibited significantly decreased invasion into or being uptaken by A. castellanii as compared to the invasive E. coli K1
Next, to determine the E. coli intracellular of amoebae, the invasion assay was performed. We observed a higher recovery (0.008 bacterial colony per amoeba) of E. coli K1 intracellular of A. castellanii as compared to K12 (no bacterial colony per amoeba) for 1 hr (Fig. 2) (P<0.05). Interestingly, E. coli K5 was recovered less (0.00011 bacterial colonies per amoeba) than E. coli K1 but still alive inside A. castellanii for 1 hr (P<0.05).
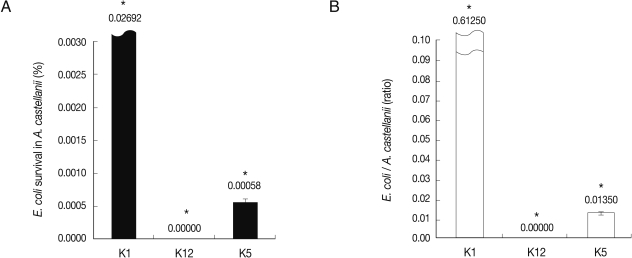
E. coli K5 survived intracellularly in A. castellanii while non-invasive E. coli K12 were killed
To determine the fate of E. coli in long-term interactions with A. castellanii, the intracellular survival assay was performed by incubating E. coli with Acanthamoeba in PBS for 24 hr. With regard to the clinical isolates of Acanthamoeba, our findings revealed that once intracellular, E. coli K1 remained viable and multiplying, while K12 were killed (Fig. 3) (P<0.05). Interestingly, E. coli K5 survived and multiplied a little intracellularly in A. castellanii. It is suggested that intracellularly invaded E. coli K5 multiply in the cytoplasm of A. castellanii.
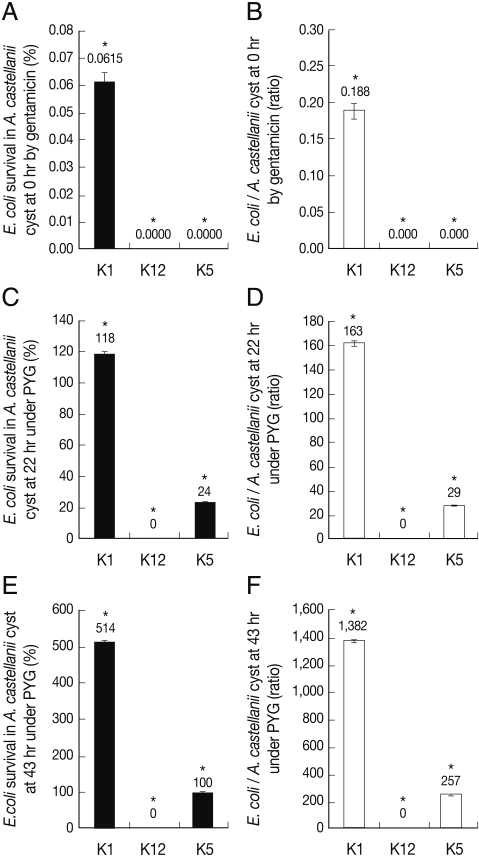
E. coli K5 survived inside the cyst form of A. castellanii as compared with E. coli K1
After A. castellanii was incubated with bacteria for 1 hr and then treated with gentamicin for 45 min, all Acanthamoeba trophozoites were inoculated onto non-nutrient agar plate to induce encystment of A. castellanii. The encystment was observed periodically up to 43 hr. The groups of A. castellanii cysts were grape-shaped as well as tightly linked (Fig. 4). Invasive E. coli K1 survived intracellularly and/or extracellularly in A. castellanii at 0 hr (0.052 bacterial colony per amoeba; 0.044 cfu per amoeba) (Fig. 5) (P<0.05). Otherwise, E. coli K12 and E. coli K5 didn't survive intracellularly and/or extracellularly in A. castellanii at 0 hr. To determine whether a very few number of E. coli K5 may survive intracellularly in A. castellanii, cyst forms of A. castellanii were incubated under a favourable condition of PYG medium up to 43 hr, which would exhibit transformation of cysts into trophozoites and bacteria might come out of A. castellanii trophozoites. Under the favourable condition, no non-invasive E. coli K12 were come up but E. coli K5 multiplied significantly up to estimated 43 hr. In addition, the number of A. castellanii trophozoites was not significantly increased during 22 hr and 43 hr more than 0 hr (data not shown). Very interestingly, E. coli K5 multiplied and survived in transformed A. castellanii trophozoites under favourable conditions, suggesting that E. coli K5 in A. castellanii cysts be alive and come out of trophozoites under favourable conditions.
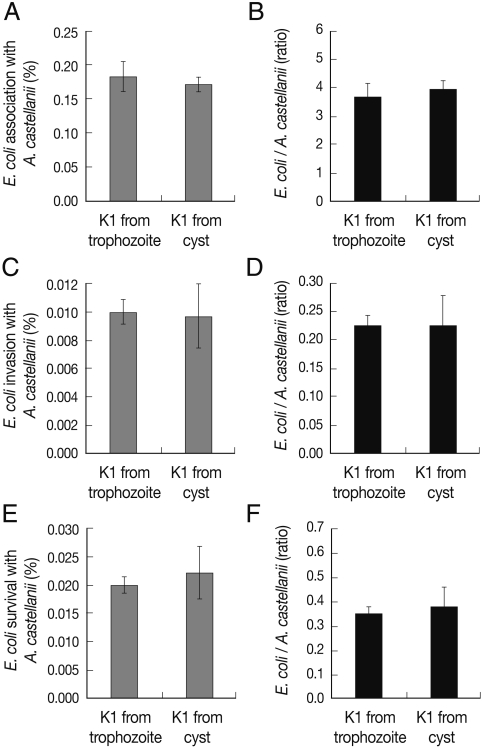
Pathogenic potential of E. coli K1 from A. castellanii cysts exhibited no changes as compared with E. coli K1 from A. castellanii trophozoites
To determine whether invasive E. coli K1 from A. castellanii trophozoites may have different pathogenic potential from invasive E. coli K1 from A. castellanii cysts, the association, invasion, and survival assays were performed as mentioned above. The ability of E. coli K1 from A. castellanii cysts to interact with A. castellanii trophozoites exhibited little changes as compared with E. coli K1 from A. castellanii trophozoites to interact with A. castellanii trophozoites (Fig. 6). These results revealed that although the intracellularly survived E. coli K1 from A. castellanii cysts multiplied, the pathogenic potential of the E. coli K1 might have never changed.
DISCUSSION
The predatory role of Acanthamoeba in the control of bacterial populations and their ability to host bacteria and/or to act as a reservoir for bacterial pathogens suggests that these protists play important roles in the environment and contribute to human infections with bacteria. For the latter, a clear evidence is that the environmental bacterium, L. pneumophila parasitize Acanthamoeba as well as use it as a host or a vehicle to infect the human lung macrophages. The ability of L. pneumophila to survive intracellularly in Acanthamoeba has been suggested to be a key step in the evolution of this environmental bacterium to produce human infections. This concept is further strengthened with the finding that Acanthamoeba resembles human macrophages in many ways, particularly in their phagocytic activity and cell surface receptors [21] and that macrophages and Acanthamoeba exhibit parallel mechanisms in their interactions with L. pneumophila [22].
Here, to determine the differential interactions of E. coli K5 and E. coli K1 with A. castellanii, we analyzed association with, invasion into, and survival of these bacteria in A. castellanii. In previous studys, E. coli K1 of other capsular antigen compared with E. coli K12 were highly associated, invaded, and intracellularly survived in A. castellanii [1,8]. Interestingly, LPS chemical mutant of E. coli K1 exhibited less association, invasion, and survival in A. castellanii as compared with invasive E. coli K1. Beside FimH, both OmpA and LPS were crucial in E. coli K1 binding, association, and subsequent survival and replication within A. castellanii tested. This finding showed remarkable similarities with human macrophages. For example, recent studies have shown that E. coli K1 but not K12 are able to enter, survive, and replicate intracellularly in murine and human macrophages [23]. This property may be crucial for E. coli K1 survival in the bloodstream, a primary step in the development of meningitis.
As shown in this study, E. coli K5 exhibited significantly low association, invasion, and intracellular survival in A. castellanii trophozoites as compared with E. coli K1. It survived in A. castellanii cysts. Although A. castellanii cysts were transformed into trophozoites under PYG media, no E. coli K5 survived up to 0 hr which indicated the invasion assay. Thereafter, E. coli K5 survived in A. castellanii cysts and also recovered in transformed A. castellanii trophozoites under favourable conditions. Taken together, they implied that A. castellanii trophozoites more active in phagocytosis and movement than cysts would engulf E. coli K5. In fact, E. coli K1 differs from E. coli K5 by the somatic antigen but not capsular antigen. E. coli K5 showed less association, invasion, and survival as compared with E. coli K1 with A. castellanii trophozoites and cysts. More importantly, the pathogenic potential of E. coli K1 was not changed as shown in Fig. 6.
Previous studies have shown that the attachment of L. pneumophila to the protozoan host, Hartmanella vermiformis is mediated by galactose/N-acetylgalactoseamine residues on the surface of bacteria that are recognized by a 170 kDa receptor lectin on the surface of protozoa [24,25]. In contrast, adherence of L. pneumophila to macrophages is dependent on heat-labile serum opsonins [26]. However, the mechanisms of subsequent survival and replication of L. pneumophila within protozoa and macrophages are remarkably similar [22,27-30]. The fact that Acanthamoeba resembles human macrophages in many ways, particularly in their phagocytic activity [9], and that macrophages and Acanthamoeba exhibit parallel mechanisms in their interactions with E. coli, suggest that Acanthamoeba may provide an alternative model to study E. coli pathogenesis as well as to understand bacterial immune evasion strategies. Future studies will establish this hypothesis and will precisely determine whether E. coli K5 survival and multiplication intracellularly of Acanthamoeba affects its virulence.
Overall, these studies suggest that E. coli K5 interactions with A. castellanii trophozoites are not significantly increased as compared with E. coli K1. Otherwise, E. coli K5 interactions were very similar with non-invasive E. coli K12. However, the interaction of E. coli K5 and A. castellanii cysts was obviously different from that of E. coli K12. Therefore, although the capsular serotypes were identical, these results suggested that the different somatic antigen in E. coli K5 would be a very important role in association, invasion, and survival of E. coli K5 in A. castellanii trophozoites and cysts.