AbstractTo evaluate the efficacy of ronidazole for treatment of Tritrichomonas foetus infection, 6 Tritrichomonas-free kittens were experimentally infected with a Korean isolate of T. foetus. The experimental infection was confirmed by direct microscopy, culture, and single-tube nested PCR, and all cats demonstrated trophozoites of T. foetus by day 20 post-infection in the feces. From day 30 after the experimentally induced infection, 3 cats were treated with ronidazole (50 mg/kg twice a day for 14 days) and 3 other cats received placebo. Feces from each cat were tested for the presence of T. foetus by direct smear and culture of rectal swab samples using modified Diamond's medium once a week for 4 weeks. To confirm the culture results, the presence of T. foetus rRNA gene was determined by single-tube nested PCR assay. All 3 cats in the treatment group receiving ronidazole showed negative results for T. foetus infection during 2 weeks of treatment and 4 weeks follow-up by all detection methods used in this study. In contrast, rectal swab samples from cats in the control group were positive for T. foetus continuously throughout the study. The present study indicates that ronidazole is also effective to treat cats infected experimentally with a Korean isolate of T. foetus at a dose of 50 mg/kg twice a day for 14 days.
Tritrichomonas foetus is a flagellated protozoan parasite that colonizes the ileum, cecum, and colon, causing colitis and chronic large bowel diarrhea in cats [1]. Infected cats may have persistent diarrhea as long as 2 years and may remain infected for life, although spontaneous resolution of diarrhea may occur [2]. In the Republic of Korea, the first clinical case of feline intestinal tritrichomoniasis was reported in our previous study [3]. We also found that 6.8% (5/73) of surveyed cats in Korea were infected with T. foetus (paper submitted).
To date, the only known effective drug for T. foetus is ronidazole [1], which is currently not approved for use in companion animals [4]. However, the chemical-grade powder is available and the drug can be formulated into either tablets or capsules by pharmacies [5]. Ronidazole, a 5-nitroimidazole, killed T. foetus in vitro [6] and eliminated T. foetus from experimentally infected cats at doses ranging from 30 to 50 mg/kg orally twice a day for 14 days [1]. At lower doses (10 mg/kg PO orally twice a day for 14 days), ronidazole has been ineffective at eliminating T. foetus infection [1]. The purpose of this study was to evaluate the efficacy of ronidazole for treatment of cats experimentally infected with a Korean isolate of T. foetus, on the basis of culture and PCR testing of rectal swab samples.
T. foetus was originally isolated from a naturally infected female Siamese cat in our previous study [3]. Trophozoites were successfully subcultured in modified Diamond's medium (Remel, Lenexa, Kansas, USA) in the laboratory and the Korean isolate (YG 1) was used for experimental inoculation in this study.
Five to seven month-old female (n=2) and male (n=4) domestic Korean shorthair cats were purchased from a local market for experimental infection with T. foetus. Each cat was confirmed to be free of T. foetus and Giardia spp. by direct fecal smear, rectal swab culture in modified Diamond's medium, and a single-tube nested PCR assay as described previously [7]. All cats were additionally examined for the presence of Giardia-specific antigen using commercial Giardia antigen test kit (SNAP™ Giardia, IDEXX Laboratories, Westbrook, Maine, USA).
Cats were randomly divided into 2 equal groups; 3 for treatment and 3 for control. Each cat was sedated with acepromazine maleate (0.05 mg/kg, IM), atropine sulfate (0.04 mg/kg, SC), and a combination of tiletamine and zolazepam (5 mg/kg, IM), and received 360 µl of media containing approximately 2,000,000 live T. foetus trophozoites through a feeding tube. Infection with T. foetus was confirmed by direct microscopy and single-tube nested PCR testing as described previously [7]. After 7 days of oral inoculation, rectal swab samples of each cat were suspended in saline and examined microscopically daily until motile trophozoites of T. foetus were observed. All 6 cats demonstrated trophozoites of T. foetus by day 20 post-infection (PI) in the feces.
From day 30 after the experimentally induced infection, treatment with ronidazole was initiated. Three cats in the treatment group were treated with oral administration of ronidazole at 50 mg/kg twice a day for 14 days, and 3 other cats belonging to the control group received empty capsules. Chemical grade ronidazole ([1-methyl-5-nitroimidazole-2-yl]-methyl carbamate; 99% pure, R7635-5G; Sigma Chemical Co, St. Louis, Missouri, USA) was compounded into gel capsules for use in this study. Fecal samples from each cat were tested for the presence of T. foetus trophozoites by direct smear and culture of rectal swab samples using modified Diamond's medium (Remel Media; Remel) once a week for 4 weeks. To confirm the culture results, the presence of T. foetus rRNA gene was determined by single-tube nested PCR assay using a combination of TFITS-F/TFITS-R and TFR3/TFR4 primers as described previously [7].
All cats were confirmed to be free of T. foetus before experimentally induced infection by direct smear and single-tube nested PCR test of rectal swab samples (data not shown). Some of the cats started to reveal T. foetus trophozoites in rectal samples at day 7 after inoculation, and all cats were positive by day 20 PI (Table 1; Fig. 1A). No cat showed signs of diarrhea after experimentally induced infection in this study.
All 3 cats in the treatment group receiving ronidazole showed negative results for T. foetus infection during 2 weeks of treatment and 4 weeks follow-up by all detection methods used in this study. In contrast, rectal swab samples from each untreated cat (control group) were positive for T. foetus continuously throughout the study (Table 2; Fig. 1B).
Currently, there is no effective commercially-available drug against intestinal T. foetus infection in cats. Metronidazole has often been used as an initial choice of drug against feline intestinal trichomoniasis, but organisms were hardly eliminated, and diarrhea resumed shortly after the last medication with the drug [1]. Ronidazole is reported to be the only known effective treatment for T. foetus [8]. Oral administration of ronidazole at 30-50 mg/kg twice a day for 14 days resolved diarrhea and eradicated T. foetus [1]. We therefore evaluated the efficacy of ronidazole at 50 mg/kg twice a day for 14 days for treatment of cats experimentally infected with a Korean isolate of T. foetus.
As has been reported previously [1], cats experimentally infected with a Korean isolate of T. foetus were also successfully treated with ronidazole at 50 mg/kg twice a day for 14 days. Trophozoites were not detected from cats in the treatment group by all detection methods used in this study, including PCR assay from 1 week post-treatment, whereas cats belonging the control group showed T. foetus-positive continuously throughout the study period after infection. However, the duration of follow-up in the present study was much shorter than that of the previous study [1], in which repeated PCR testing was performed over durations of 21-30 weeks to confirm eradication of the infection in cats. The authors observed that infection could relapse as long as 20 weeks after the treatment, and thus short-term studies of drug efficacy in T. foetus infected cats should be viewed with caution [1].
Unfortunately, we were unable to observe the effects of ronidazole treatment on diarrhea in the experimentally infected cats, because diarrhea was not observed from any of the 6 cats before or during the treatment period. The reason for the absence of diarrhea in the cats is not known, but this observation is consistent with previous studies on experimental infections [1,8,9]. Gookin et al. [8] suggested the possibility of strain differences in the pathogenicity of T. foetus, as has been reported for Trichomonas vaginalis [8,10]. Further studies are needed regarding the mechanisms of the pathogenicity of T. foetus in cats.
Another possibility is the difference in susceptibility by breed of cats. According to some previous studies, pedigree cats appear to be more frequently infected with T. foetus than mixed-bred cats [11,12], although many other researchers did not describe the same conclusion [12-15]. In our previous study on the prevalence of T. foetus in cats in Korea, we found only 1.9% (1/53) of non-pedigree domestic cats was positive, while 50% (2/4) and 11% (1/9) of Siamese and Persian breeds of cats were positive, respectively (paper submitted). However, it is difficult to draw conclusions regarding the association between T. foetus and breed of cats in the study because of the small number of cats surveyed. A high rate of T. foetus infection among specific breeds of cats, including Siamese cats, was also reported in a previous study in the UK [11].
The present study indicates that ronidazole is effective to treat cats infected experimentally with a Korean isolate of T. foetus at a dose of 50 mg/kg twice a day for 14 days. Although no reports are available on the differences in susceptibility by different isolates to chemotherapy, the potential presence of different strains/isolates and a rather narrow choice of drug to treat feline tritrichomoniasis indicate that the susceptibility of the Korean isolates of T. foetus to ronidazole needs to be evaluated.
ACKNOWLEDGMENTThis work was supported in part by the Ministry of Education and Human Resources Development through the Brain Korea 21 Project in Korea.
REFERENCES1. Gookin JL, Copple CN, Papich MG, Poore MF, Stauffer SH, Birkenheuer AJ, Twedt DC, Levy MG. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med 2006;20:536-543. PMID: 16734086.
2. Foster DM, Gookin JL, Poore MF, Stebbins ME, Levy MG. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc 2004;225:888-892. PMID: 15485048.
3. Lim S, Park SI, Ahn KS, Oh DS, Ryu JS, Shin SS. First report of feline intestinal trichomoniasis caused by Tritrichomonas foetus in Korea. Korean J Parasitol 2010;48:247-251. PMID: 20877505.
4. LeVine DN, Papich MG, Gookin JL, Davidson GS, Davis JL, Hayes RB. Ronidazole pharmacokinetics after intravenous and oral immediate-release capsule administration in healthy cats. J Feline Med Surg 2011;13:244-250. PMID: 21239199.
5. Tolbert MK, Gookin J. Tritrichomonas foetus: A new agent of feline diarrhea. Compend Contin Educ Vet 2009;31:374-381. PMID: 19866444.
6. Gookin JL, Stauffer SH, Dybas D, Cannon DH. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med 2010;24:1003-1007. PMID: 20492492.
7. Gookin JL, Birkenheuer AJ, Breitschwerdt EB, Levy MG. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol 2002;40:4126-4130. PMID: 12409385.
8. Gookin JL, Stauffer SH, Coccaro MR, Poore MF, Levy MG, Papich MG. Efficacy of tinidazole for treatment of cats experimentally infected with Tritrichomonas foetus. Am J Vet Res 2007;68:1085-1088. PMID: 17916015.
9. Gookin JL, Levy MG, Law JM, Papich MG, Poore MF, Breitschwerdt EB. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res 2001;62:1690-1697. PMID: 11703009.
10. Gómez-Barrio A, Nogal-Ruiz JJ, Montero-Pereira D, Rodríguez-Gallego E, Romero-Fernández E, Escario JA. Biological variability in clinical isolates of Trichomonas vaginalis. Mem Inst Oswaldo Cruz 2002;97:893-896. PMID: 12386717.
11. Gunn-Moore DA, McCann TM, Reed N, Simpson KE, Tennant B. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg 2007;9:214-218. PMID: 17446107.
12. Stockdale HD, Givens MD, Dykstra CC, Blagburn BL. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol 2009;160:13-17. PMID: 19070434.
13. Frey CF, Schild M, Hemphill A, Stünzi P, Müller N, Gottstein B, Burgener IA. Intestinal Tritrichomonas foetus infection in cats in Switzerland detected by in vitro cultivation and PCR. Parasitol Res 2009;104:783-788. PMID: 18998166.
14. Burgener I, Frey C, Kook P, Gottstein B. Tritrichomonas fetus: a new intestinal parasite in Swiss cats. Schweiz Arch Tierheilkd 2009;151:383-389. PMID: 19653162.
15. Kuehner KA, Marks SL, Kass PH, Sauter-Louis C, Grahn RA, Barutzki D, Hartmann K. Tritrichomonas foetus infection in purebred cats in Germany: prevalence of clinical signs and the role of co-infection with other enteroparasites. J Feline Med Surg 2011;13:251-258. PMID: 21288749.
Fig. 1Single-tube nested PCR amplification (208-bp) of Tritrichomonas foetus with primers TFR3/TFR4 and primers TFITS-F/TFITS-R in cats from Korea on day 0 (A) and day 28 after the initiation of treatment with ronidazole at 50 mg/kg twice a day for 14 days (B). Lane 1, molecular weight marker; 2, positive control; 3, culture medium; 4, 5, 6, untreated control cats (C-16, C-70, C-71); 7, 8, 9, cats treated with ronidazole (C-30, C-45, C-77). 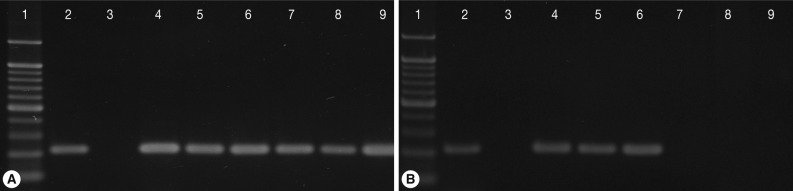
Table 1.Confirmation of experimental oral inoculation of cats with Tritrichomonas foetus by direct smear examination of rectal swab samples
Table 2.Efficacy of ronidazole against experimental infection of cats with a Korean isolate (YG 1) of Tritrichomonas foetus
A direct smear and culture of rectal swab samples were carried out once a week during the 2 weeks of treatment period and for 4 weeks after the termination of the last treatment. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||