AbstractNatural habitat fragmentation and reducing habitat quality have resulted in an increased appearance of Japanese macaques, Macaca fuscata (Gray, 1870), in suburban areas in Japan. To investigate the risk of zoonotic infections, a coprological survey of helminth eggs passed by wild Japanese macaques was carried out in 2009 and 2010 in Shiga Prefecture, Japan. Microscopic examination found helminth eggs in high prevalence, and nucleotide sequencing of DNA extracted from the eggs identified Oesophagostomum cf. aculeatum and Trichuris trichiura. A fecal culture also detected infective larvae of Strongyloides fuelleborni. These zoonotic nematodes pose a potential health issue to local people in areas frequented by Japanese macaques.
The Japanese macaque, Macaca fuscata (Gray, 1870), is unique to the Japanese archipelago and is the only non-human primate that inhabits these islands, with an estimated total population of 100,000 [1]. Advancing forest habitat fragmentation and reducing habitat quality in recent years have forced Japanese macaques to appear frequently at forest edges and in farmland near residential areas. Japanese macaques carry some helminths potentially zoonotic to humans, including nematodes, such as Oesophagostomum aculeatum, Strongyloides fuelleborni, and Trichuris trichiura [1,2]. Traditionally, however, specific diagnosis of gastrointestinal nematode infections in wild non-human primates is based on the detection of eggs in feces, where the morphological characteristics of ova alone may not be reliable enough for species identification. Thus, to identify the helminths carried by wild Japanese macaques, and to evaluate the risk of zoonotic infections to humans, we carried out molecular identification of eggs passed in feces by sequencing some nuclear targets.
Fecal samples were collected in May 2009 and June 2010 from the ground at the foot of mountains in Takashima city, Shiga Prefecture, Japan. This district is a rather sparsely-populated area adjacent to farmland that is frequented by free-roaming macaques at irregular intervals. That the fecal samples were derived from Japanese macaques was confirmed by local residents who had often seen monkeys and their feces at very close proximity to their houses. A total of 36 fecal samples were collected in 2009 and 2010. Although the length of time the feces had remained on the ground could not be determined, they appeared to have been there only for a few days.
The fecal samples, 2 g each, were dissolved in 200 ml of distilled water, sieved through 2 sheets of cotton gauze, and left to stand for 1-2 hr to obtain precipitates. After 2 additional cycles of dissolution and sieving, the precipitates finally obtained were subjected to microscopic examination for helminth eggs. One fecal sample was subjected to fecal culture using a filter paper test-tube method.
To molecularly identify helminth eggs, 10-30 helminth eggs (Oesophagostomum sp. or Trichuris sp.) were collected under a stereoscopic microscope. Approximately 50 Strongyloides filariform larvae were collected from the bottom of culture test tubes. DNA was extracted using TaKaRa DEXPAT® (Takara Bio Inc., Shiga, Japan), which was originally designed to retrieve small amounts of DNA from paraffin embedded tissue sections. The primers used for PCR amplification were: NC1 and NC2 [3] for internal transcribed spacer (ITS)2 of Oesophagostomum spp.; 5'-CAAGGTTTTCGTAGGTGAA-3' and 5'-CTCTTCATCGACCTATGAAC-3' for ITS1 of Strongyloides spp.; and 5'-CTAAGCAGAGCCTTAAATT-3' and 5'-TCCGCTTAACGATATGCTTA-3' for ITS2 of Strongyloides spp. The 18S rRNA of Trichuris spp. was amplified in 4 overlapping fragments using the forward and reverse primers 5'-AAGCCGCGAATGGCTCATTA-3' and 5'-CTGCTGCCTTCCTTGGATGT-3' for the first fragment, 5'-CCATGGTGACAACGGTTAAC-3' and 5'-ATTGGTCGTCTTGCTGCGAT-3' for the second fragment, 5'-ACGGGGACATTCGTATTGCT-3' and 5'-GCTAGTTAGTAGGCCAGAGT-3' for the third fragment, and 5'-TTCAGTGGGTAGTGGTGCAT-3' and 5'-CCTACGGAAACCTTGTTACG-3' for the last fragment. The amplified products were directly sequenced on both strands. The nucleotide sequences determined in this study were deposited in DNA databases with the accession numbers indicated in the text, Table, and/or Figures.
Microscopic examination revealed eggs similar to those of hookworms and eggs of Trichuris sp. in 50% and 38% of 18 fecal samples examined in 2009, and 38% and 31% of 18 fecal samples examined in 2010. Eggs similar to those of Strongyloides sp. were also found in a few fecal samples in both years. Fecal cultures of 1 specimen collected in 2010 produced filariform larvae of Strongyloides sp. Cysts of Entamoeba coli were also occasionally found.
Hookworm-like eggs found in the present survey were thin-shelled, ovoidal, segmented into ≥8 cells, and measured 69-78 by 41-48 (mean: 74×45) µm (Fig. 1A). Non-human primates harbor strongylid nematodes, such as Oesophagostomum spp. and Ternidens spp., the eggs of which are hardly distinguishable from those of hookworms. Within these species, O. aculeatum is confined to Asia and infects primates, such as Japanese macaques and cynomolgus monkey (Macaca fascicularis) [4-7], while Oesophagostomum bifurcum and Oesophagostomum stephanostomum infect African primates as well as rhesus monkeys (Macaca mulatta) in Asia [3,6,8,9]. Ternidens deminutus occurs in primates in Asia and Africa [5,10,11], but has not been recorded in Japanese macaques. Thus, the type of ova found in the present study has been traditionally identified as that of O. aculeatum, as long as it was derived from Japanese macaques [1,2]. Indeed, the dimensions of the hookworm-like eggs found in the present study were compatible with those of O. aculeatum (69-86 by 35-55 µm), the measurements of which were based on intrauterine eggs from O. aculeatum female worms, but were larger than those of T. deminutus (57-65 by 36-45 µm) [4].
ITS2 sequencing was carried out in 2 specimens containing hookworm-like eggs collected in 2009 and 2010. Sequences obtained in both specimens were identical and deposited in public databases (AB586134). Unfortunately, we could neither obtain morphologically identified O. aculeatum adult worms for sequencing, nor find any reference sequence of O. aculeatum in the literature or DNA databases. Nevertheless, a BLAST search against the EMBL databases identified sequences of Oesophagostomum sp. from M. fuscata (HM067976) with 99.0% identity, followed by those of O. bifurcum and other Oesophagostomum species with identities≤94.0%. Pairwise comparisons of ITS2 sequences showed that the putative O. aculeatum from Japanese macaques were clearly distinguished from O. bifurcum and O. stephanostomum with genetic distances equal to or over 0.047, and from T. deminutus with genetic distances of ≥0.056 (Table 1). Thus, we preliminarily identified the eggs as Oesophagostomum cf. aculeatum until it could be verified by sequencing nucleotides of morphologically identified O. aculeatum adult worms.
Trichuris eggs obtained in the present study measured 58-63×28-31 (mean: 61×29) µm (Fig. 1B). The eggs of human T. trichiura are divided into 2 morphotypes; the common type with a dimension of 56±2 by 26±1 µm, and the less common type with larger dimensions (75±4 by 31±2 µm) [12]. The eggs of Japanese macaque-origin were similar to those of the common variety of T. trichiura eggs. Sequencing of 18S rRNA in Trichuris eggs from Japanese macaques and in 2 adult worms of T. trichiura from 2 Japanese patients as a reference was carried out. Pairwise comparisons showed that Trichuris derived from Japanese macaques (AB699092) differed from T. trichiura from 2 Japanese patients (AB699090) and another T. trichiura worm of human origin (GQ352553) with genetic distances of 0.003 and 0.005, respectively. Its genetic distances from Trichuris suis, Trichuris vulpis, and Trichuris muris were ≥0.028. Phylogenetic analyses showed that Trichuris eggs from Japanese macaques clustered unequivocally with T. trichiura of human origin (Fig. 2). From these results, we identified the eggs as T. trichiura.
Filariform larvae of Strongyloides sp. produced in a fecal culture of monkey feces were 502-603 µm in length with a body width of 13-16 µm. The esophagus accounted for 40-45% of body length. The tail measured 66-82 µm in length. ITS1 sequences of the filariform larvae obtained in the present study (AB699093) were similar to those of S. fuelleborni (AB272235, U43581), with genetic distances of 0.013-0.019, while they showed larger differences from Strongyloides callosciureus (AB272229), Strongyloides procyonis (AB205054), Strongyloides stercoralis (U43578), Strongyloides ratti (U43580), and Strongyloides cebus (AB272236), with genetic distances of ≥0.165. ITS2 sequences of the present isolates (AB699094) were also similar to those of S. fuelleborni (AB272235) with a genetic distance of 0.009. Thus, the filariform larvae in the present study were identified as S. fuelleborni.
The present coprological survey of wild Japanese macaques detected eggs of Oesophagostomum. cf. aculeatum and T. trichiura at a rather high prevalence, as well as infective larvae of S. fuelleborni, confirming previous reports that the eggs of O. aculeatum, T. trichiura, and S. fuelleborni were found in 30.4%, 60.1%, and 29.0% of Japanese macaques, respectively [1].
Oesophagostomum infections in humans result in single or multiple nodular lesions mainly in the colon and sometimes in extraintestinal tissues. A number of O. bifurcum infections in humans have been reported, with the majority from Africa, especially northern Togo and Ghana [9,13]. A few cases of possible O. aculeatum infections have also been reported in Southeast Asia [14-16]. Except for those cases in Togo and Ghana, larvae often do not complete development in the human body and juveniles remain in their intestinal nodules, which makes diagnosis difficult. The infection of humans with Oesophagostomum spp. is considered to occur via ingestion of the 3rd-stage larvae in contaminated water, food, and soil. Although no Oesophagostomum infection in humans has been reported in Japan, people should be aware of the risk of accidental infection.
It has been widely accepted that the Trichuris species, which infects non-human primates, is similar to that in man and cross infection to humans is possible [6]. Indeed, previous studies showed that experimental infection of man with Trichuris ova from Japanese macaques resulted in patent infection [17]. The present phylogenetic analyses based on 18S rRNA sequences showed a very close relationship of Trichuris of macaque-origin to that of human-origin, with minor genetic divergence. Previous studies by scanning electron microscopy showed that T. trichiura from non-human primates exhibited some differences in pericloacal papillae from those of human origin; although the authors considered the findings insufficient to create a new species [18]. Thus, it remains to be elucidated whether population genetic substructuring exists within T. trichiura from different primate hosts by analyzing such as mitochondrial DNA sequences, and more studies are needed to clarify whether T. trichiura of macaque-origin accounts for at least some cases of human trichuriasis.
S. fuelleborni is zoonotic: it has been reported that 3 out of 10 cases of human strongyloidiasis in Zambia were due to S. fuelleborni [19-21]. However, genetic substructuring of S. fuelleborni exists worldwide according to geographical localities [21], and further studies are needed to clarify whether infectivity of S. fuelleborni of Japanese-macaque origin to humans is similar to those from different localities.
Besides those helminth ova found in the present study, eggs of the tapeworm Bertiella studeri have been detected in 8.9% of fecal samples from Japanese macaques [1], and at least 5 cases of accidental infection of humans with B. studeri have occurred in Japan [22,23]. Non-human primates are also potential reservoirs for zoonotic transmission of enteric protozoans. Indeed, Giardia intestinalis (assemblage B) and Entamoeba spp., such as E. dispar and E. nuttalli, have been isolated from Japanese macaques [24-26].
In conclusion, the present coprological survey of wild Japanese macaques identified Oesophagostomum cf. aculeatum, T. trichiura, and S. fuelleborni based on sequencing nuclear targets. Further accumulation of sequence data will lead to the identification of these parasites from Japanese macaques based upon more solid evidence. Although further studies are needed to determine the actual risk of zoonotic transmissions of helminths between Japanese macaques and humans, people and health workers should be aware of the potential risk of accidental zoonotic helminth infections in humans living in areas frequented by Japanese macaques.
REFERENCES1. Fooden J, Aimi M. Systematic review of Japanese macaques, Macaca fuscata (Gray, 1870). Fieldiana Zoology new series, no.104. 2005, Chicago, USA. Field Museum of Natural History. pp 1-200.
2. MacIntosh AJ, Hernandez AD, Huffman MA. Host age, sex, and reproductive seasonality affect nematode parasitism in wild Japanese macaques. Primates 2010;51:353-364. PMID: 20711744.
3. Gasser RB, Woods WG, Huffman MA, Blotkamp J, Polderman AM. Molecular separation of Oesophagostomum stephanostomum and Oesophagostomum bifurcum (Nematoda: Strongyloidea) from non-human primates. Int J Parasitol 1999;29:1087-1091. PMID: 10501618.
4. Tanaka H, Fukui M, Yamamoto H, Hayama S, Kodera S. Studies on the identification of common intestinal parasites of primates. Bull Exp Anim 1962;11:111-116 (in Japanese).
5. Hashimoto I, Honjo S. Survey of helminth parasites in cynomologus monkeys (Macaca irus). Japan J Med Sci Biol 1966;19:218. PMID: 5297027.
6. Flynn RJ. Parasites of Laboratory Animals. 1973, Ames, USA. Iowa State University Press. pp 212-214.
7. Dewit I, Dittus W, Gibson D, Harris E, Vercruysse J. Gastro-intestinal helminths in a natural population of Macaca sinica and Presbhtis spp. at Polonnaruwa, Sri Lanka. Primates 1991;32:391-395.
9. Gasser RB, de Gruijter JM, Polderman AM. Insights into the epidemiology and genetic make-up of Oesophagostomum bifurcum from human and non-human primates using molecular tools. Parasitology 2006;132:453-460. PMID: 16332292.
10. Schindler AR, de Gruijter JM, Polderman AM, Gasser RB. Definition of genetic markers in nuclear ribosomal DNA for a neglected parasite of primates, Ternidens deminutus (Nematoda: Strongylida) - diagnostic and epidemiological implications. Parasitology 2005;131:539-546. PMID: 16174419.
11. Hemsrichart V. Ternidens deminutus infection: First pathological report of a human case in Asia. J Med Assoc Thai 2005;88:1140-1143. PMID: 16404847.
12. Yoshikawa H, Yamada M, Matsumoto Y, Yoshida Y. Variations in egg size of Trichuris trichiura. Parasitol Res 1989;75:649-654. PMID: 2771930.
13. Polderman AM, Blotkamp J. Oesophagostomum infections in humans. Parasitol Today 1995;11:451-456. PMID: 15275382.
14. Ross RA, Gibson DI, Harris EA. Cutaneous oesophagostomiasis in man. Trans R Soc Trop Med Hyg 1989;83:394-395. PMID: 2617588.
15. Karim N, Yang CO. Oesophagostomiasis in man: Report of the first Malaysian case with emphasis on its pathology. Malays J Pathol 1992;14:19-24. PMID: 1469913.
16. Kamel AGM, Yang CO, Norazah A. Oesophagostomum aculeatum, the nodular worm causing helminthoma in man in Malaysia. Int Med J 1995;2:287-290.
17. Imada I, Horii Y, Usui M. The experiment of artificial infection to men and monkeys by eggs of Trichuris trichiura from Japanese monkey. Jpn J Parasitol 1986;29:S30 (in Japanese).
18. Ooi HK, Tenora F, Itoh K, Kamiya M. Comparative study of Trichuris trichiura from non-human primates and from man, and their differences with T. suis. J Vet Med Sci 1993;55:363-366. PMID: 8357906.
19. Hira PR, Patel BG. Strongyloides fülleborni infections in man in Zambia. Am J Trop Med Hyg 1977;26:640-643. PMID: 889005.
20. Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P. Strongyloidiasis - the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 2009;103:967-972. PMID: 19328508.
21. Hasegawa H, Sato H, Fujita S, Nguema PP, Nobusue K, Miyagi K, Kooriyama T, Takenoshita Y, Noda S, Sato A, Morimoto A, Ikeda Y, Nishida T. Molecular identification of the causative agent of human strongyloidiasis acquired in Tanzania: Dispersal and diversity of Strongyloides spp. and their hosts. Parasitol Int 2010;59:407-413. PMID: 20621633.
22. Ando K, Ito T, Miura K, Matsuoka H, Chinzei Y. Infection of an adult in Mie Prefecture, Japan by Bertiella studeri. Southeast Asian J Trop Med Public Health 1996;27:200-201. PMID: 9031429.
23. Yamada M, Uchikawa R, Tegoshi T, Onishi K, Arizono N, Kawahara A, Yamamoto H, Kodama T, Morita Y, Tashiro K. Two cases of human infection with Bertiella studeri in Japan. Clin Parasitol 2009;20:34-36 (in Japanese).
24. Itagaki T, Kinoshita S, Aoki M, Itoh N, Saeki H, Sato N, Uetsuki J, Izumiyama S, Yagita K, Endo T. Genotyping of Giardia intestinalis from domestic and wild animals in Japan using glutamete dehydrogenase gene sequencing. Vet Parasitol 2005;133:283-287. PMID: 16029929.
25. Tachibana H, Cheng XJ, Kobayashi S, Matsubayashi N, Gotoh S, Matsubayashi K. High prevalence of infection with Entamoeba dispar, but not E. histolytica, in captive macaques. Parasitol Res 2001;87:14-17. PMID: 11199843.
26. Tachibana H, Yanagi T, Akatsuka A, Kobayashi S, Kanbara H, Tsutsumi V. Isolation and characterization of a potentially virulent species Entamoeba nuttalli from captive Japanese macaques. Parasitology 2009;136:1169-1177. PMID: 19635174.
Fig. 1Eggs found in the feces of Japanese macaques. (A) Oesophagostomum cf. aculeatum. (B) Trichuris trichiura. 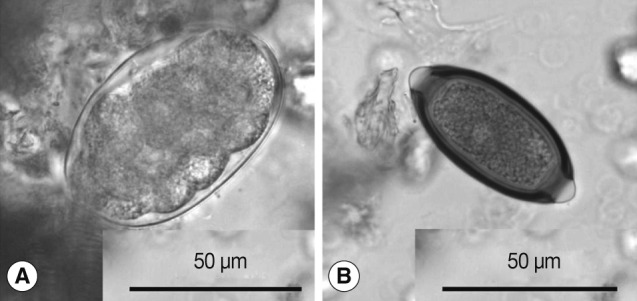
Fig. 2Phylogenetic analysis of Trichuris species based on 18S rRNA sequences. Nucleotide sequences were aligned using the ClustalW software. Phylogenetic analyses were conducted using the MEGA 5 software. Genetic relationships were inferred by the Neighbor-Joining (NJ) method using Trichinella spiralis as an outgroup. The final data set contained 1,629 positions. The scale bar indicates nucleotide substitutions per site. The Maximum Parsimony (MP) method resulted in the same topology tree, and 2 sets of bootstrap values (1,000 replicates) by the NJ and MP are shown from left to right. Trichuris Macaque represents that from Japanese macaques analyzed in the present study. 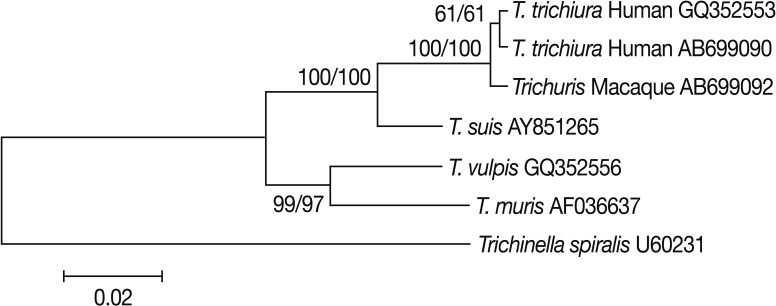
Table 1.Pairwise comparison of the number of base differencesa per site in the ITS-2 sequences of Oesophagostomum spp, Ternidens deminutus, and the 2 predominant hookworms of humans, Ancylostoma duodenale and Necator americanus
|
|
|||||||||||||||||||||||||||||||||||||