AbstractThe echinostome metacercariae encysted in Cipangopaludina sp. snails that were purchased from a market in Vientiane Municipality, Lao PDR, were identified as Echinostoma macrorchis (Digenea: Echinostomatidae) through recovery of adult flukes after experimental infection to rats and a cat. The metacercariae were round, 113-128 (121)×113-125 (120) µm, having a thick cyst wall, a head collar armed with collar spines, and excretory granules. The adult flukes recovered from the rats and cat at day 14 and 30 post-infection, respectively, were elongated, ventrally curved, and 3.9-6.3×0.7-1.1 mm in size. The head collar was distinct, bearing 43-45 collar spines with 5 angle spines on each side. Two testes were large (as the name implies), tandem, and slightly constricted at the middle, with irregular margins. Eggs were operculated, ovoid to elliptical, and 88-95×56-60 µm. In scanning electron microscopy, the head collar was prominent, with 43-45 collar spines. Scale-like tegumental spines were densely distributed on the ventral surface between the oral and ventral suckers. Sensory papillae were distributed mainly on the tegument around the 2 suckers. It is confirmed that E. macrorchis is distributed in Lao PDR using Cipangopaludina sp. snails as the second intermediate host.
INTRODUCTIONEchinostomes (Digenea: Echinostomatidae) are intestinal trematodes of humans and animals and can cause mucosal inflammation, ulceration, and bleeding in the small intestine [1]. Echinostoma, Echinochasmus, Acanthoparyphium, Artyfechinostomum, Episthmium, Himasthla, Hypoderaeum, and Isthmiophora are the 8 major genera comprising the family [1]. Echinostoma, the type and the largest genus, consists of more than 60 species reported in the literature [2]. Echinostoma macrorchis is one of the species and was described originally from naturally infected rats, Rattus rattus and Rattus norvegicus, in Japan [3]. Infections with this echinostome have been found in other rodent species and also birds in Japan [4-6]. Natural human infections were reported in Japan [7,8]. In countries other than Japan, the presence of E. macrorchis was reported only in Taiwan from rats (adults), snails (cercariae), and tadpoles (metacercariae) [9-11].
In Lao PDR, high prevalence of the liver fluke, Opisthorchis viverrini, and intestinal flukes, including Haplorchis taichui, H. pumilio, H. yokogawai, Prosthodendrium molenkampi, and Phaneropsolus bonnei, has been reported in riparian people in Vientiane Municipality, and Khammouane, Savannakhet, Saravane, and Champassak province [12-16]. However, reports on echinostome infections have been scarce. Human infections with Echinostoma revolutum, Artyfechinostomum malayanum, Echinochasmus japonicus, and Euparyphium sp. were reported recently from Khammouane province [17]. In December 2010, we detected echinostome metacercariae in a freshwater snail species, Cipangopaludina sp., purchased from a market in Vientiane Municipality and obtained adult flukes after experimental infection to rats and a cat. Here, we describe the morphologic characteristics of the flukes, including their surface ultrastructure, as observed by light and scanning electron microscopy.
MATERIALS AND METHODSWe purchased Cipangopaludina sp. snails (Fig. 1A) in a local market of Vientiane Municipality, Lao PDR. The snail seller told that these snails were caught around Vientiane area. They were transported to our laboratory on ice, and were artificially digested according to the method previously described [18]. Briefly, the shell was broken and the animal was taken out. The snail bodies were ground in a mortar with a pestle, and the ground mixture was incubated at 37℃ with artificial gastric juice containing 0.6% pepsin (1:10,000) and 0.8% HCl solution (concentrated). The digested material was filtered through a sieve and rinsed several times with PBS until the supernatant became clear. Echinostome metacercariae were isolated from the sediment with the aid of a stereomicroscope.
The metacercariae collected were morphologically observed and measured using a light microscope. Two rats (Sprague-Dawley, males, bred under clean condition) were infected orally with 100 metacercariae each and a cat (male, bred under clean condition) was infected with 125 metacercariae. The adult flukes were recovered in the small intestines of the rats and cat at days 14 and 30 post-infection (PI), respectively. Twenty adult specimens, 10 each from the rat and the cat, were fixed with 10% neutral buffered formalin under a cover slip pressure, stained with Semichon's acetocarmine, and mounted in Canada balsam. They were then observed and measured using a light microscope (LM). In the animal experiments, the guidelines of animal experiments from Gyeongsang National University School of Medicine were followed.
To observe the surface ultrastructure of the adult flukes, some worms were washed several times with 0.2 M cacodylate buffer (pH 7.2) and fixed with 2.5% glutaraldehyde at 4℃. After washing 3 times with the same buffer, they were dehydrated through a graded ethanol series (50%, 70%, 80%, 90%, 95%, and 100% alcohol), dried with hexamethyldisilazane, coated with gold using a JFC-1100E ion sputtering device (Jeol, Tokyo, Japan), and observed with a XL-30S scanning electron microscope (SEM) (Philips, Amsterdam, The Netherlands) at a 20 kV accelerating voltage.
Voucher specimens were deposited in the U.S. National Parasite Collection, Beltsville, Maryland, as USNPC 105814-105815 (1 adult worm each from an experimental rat and cat), and Parasitology Laboratory, Seoul National University College of Medicine, Seoul, Korea, as SNUPC 201271-201278 (4 adult worms from a rat and 4 from a cat).
RESULTSRecovery of metacercariae and adultsA total of 380 metacercariae of E. macrorchis were recovered from 30 Cipangopaludina sp. snails examined. The number of adult E. macrorchis specimens recovered at day 14 PI from 2 rats was 6 (6.0%) and 17 (17.0%), respectively. The number of specimens recovered at day 30 PI from the cat was 58 (46.4%). The overall worm recovery rate in 3 experimental animals during days 14-30 PI was 24.9% (81/325).
LM morphology
Metacercaria (Fig. 1B, C): The metacercarial cyst was round or slightly elliptical and 113-128 (121)×113-125 (120) µm in size (n=10), with double cyst walls of 3.8-5.0 (4.5) µm in total thickness. The head collar was distinct, enveloping the round and subterminal oral sucker, with total 43 collar spines distributed around the head collar. The ventral sucker was slightly larger than the oral sucker. Excretory granules were bead-shape concretions and filled in 2 descending canals of the main excretory tube (Fig. 1B, C).
Adult (Figs. 1D-F, 2A-G; Table 1): Adult flukes, aged 14 or 30 days, were elongated and ventrally curved stooping the anterior end, and 3.9-6.3 (5.1)×0.7-1.1 (0.9) mm in size (n=20). Specimens recovered from the cat at day 30 PI (5.4-6.3 mm long; n=10) were slightly larger than those recovered from the rat at day 14 PI (3.9-4.7 mm long; n=10). The head collar was distinct, bearing 43-45 collar spines with 5 angle group spines on each lappet of the collar. The oral sucker was subterminal, prepharynx very short, pharynx well developed, and esophagus somewhat long. The cirrus sac was elongated oval, containing a saccular seminal vesicle, prostatic gland, and cirrus briefly armed at its terminal portion, and reached beyond the middle level of the ventral sucker. The ventral sucker was large and well developed, locating at the anterior one-fifth of the body. The ovary was round and located near the median line of the body between the Mehlis' gland and uterine tubules. Two tandem testes were located near the posterior two-thirds of the body, and were characteristically large, slightly elongated, with slight indentations near the middle, having more or less irregular margins and a pointed posterior end of the posterior testis, Eggs were numerous and operculate with minute abopercular wrinkling or a knob, ovoid to elliptical, and 88-95×56-60 µm.
SEM morphologyThe adult worm was elongated and had the largest width near the middle portion of the body (Fig. 2A). The head collar was well-developed and prominent bearing total 43-45 collar spines (Fig. 2B). Angle spines were 5 in number on each side (Fig. 2B). A dorsal view of the head collar showed 2 alternating rows of collar spines (Fig. 2C). In a ventral view, a worm was stooping its anterior body and showing the dorsal collar spines (Fig. 2D). A close-up view near the anterior end of the worm showed a well-developed ventral sucker and a cirrus protruded from the genital pore with several tiny spines near its tip (Fig. 2E). Scale-like tegumental spines were densely distributed on the ventral surface between the oral and ventral suckers, with their densities decreased posteriorly (Fig. 2F). The tegument of the mid-dorsal portion of the body was devoid of spines (Fig. 2G). Sensory papillae were distributed mainly on the tegument around the 2 suckers (Fig. 2B, E).
DISCUSSIONOur specimens had more or less elongated body and dorsally uninterrupted collar spines; therefore, could be assigned to the subfamily Echinostomatinae [19]. Their head collar is well-developed with a double crown of spines and their uterine tubules are long with numerous eggs, so that they were diagnosed as a member of Echinostoma [19]. Echinostoma species of birds or mammals that have 43-45 collar spines include Echinostoma academica, E. aegyptiaca, E. attenuatum, E. australasianum, E. azerbaidjanicum, E. coromandum, E. coronale, E. dietzi, E. gotoi, E. macrorchis, and E. phasianina [20-26]. However, it was not difficult to assign our specimens to E. macrorchis based on the body length of 6.5 mm, moderately developed head collar, large and round to elliptical testes with slight indentations near the middle and with more or less irregular margins, and a pointed posterior end of the posterior testis [3,11,27].
In SEM view of our specimens, the cirrus was characteristically protruded from the genital atrium and its terminal portion was armed with several minute spines. This contrasts to a previous report in which the cirrus of E. macrorchis was stated to be unspined when observed through light microscopy [11]. It was also difficult in our study to recognize the minute spines on the tip of the cirrus by light microscopy. Anyhow, the presence of tiny spines on the terminal portion of the cirrus is a new finding in E. macrorchis adult worms. The presence or absence of spines on the cirrus has been of taxonomic significance in several species of echinostomes. For example, Echinostoma caproni [28], Echinostoma trivolvis [28], Echinostoma paraensei [29], Echinoparyphium recurvatum [30], and Himasthla limnodromi [31] lack spines on the cirrus tegument, whereas Himasthla alincia [32,33] and Acanthoparyphium tyosenense [34] have spines on the cirrus. The presence of minute spines on the cirrus tip seems to be one of the characteristic features for E. macrorchis adult flukes.
The life history of E. macrorchis has been studied in Japan [27,35-39] and Taiwan [11]. In Japan, the first intermediate host was reported to be Segmentina hemisphaerula (syn. S. mica) and Gyraulus chinensis (syn. Planorbis compressus japonicus and Gyraulus japonicus martens), whereas in Taiwan, only G. chinensis was identified to be the first intermediate host [11,36]. With regard to second intermediate hosts of E. macrorchis, various species of gastropod snails (Assiminea, Biomphalaria, Bulinus, Cipangopaludina, Gyraulus, Hippeutis, Lymnaea, Physa, Segmentina, Thiara, and Viviparus), bivalves (Corbicula), and amphibians (Hynobius and Rana) were reported in Japan and Taiwan [11,39]. In the present study, Cipangopaludina sp. snails purchased in Vientiane Municipality, Lao PDR, were proved to carry E. macrorchis metacercariae. This is the first report to prove the presence of E. macrorchis life cycle outside of Japan and Taiwan.
The natural definitive hosts of E. macrorchis have included rodents (Apodemus argenteus, A. speciosus, Microtus montebelli, Mogera spp., Rattus losea, R. norvegicus, and R. rattus) [3,5,6,9,10], birds (Capella gallinago gallinago) [4], and humans [7]. Experimental infection was successful in rats [27,35,36] and mice [40]. In the present study, it is proven that the cat is a suitable host for E. macrorchis infection. Possible presence of human infections in Lao PDR is to be determined.
Differences were noted in the morphology and life cycle of E. macrorchis between Japan and Taiwan [11]. Metacercariae of Taiwanese E. macrorchis were smaller than those of Japanese worms, 121.7±3.6 µm×118.2±4.1 µm [11] vs. 139-159 µm×99-120 µm [41]. The metacercariae from Lao PDR (in our study) were almost the same in size as those from Taiwan [11]. Whether this difference is merely a geographical variation or is taxonomically significant should be determined through further studies, including molecular genetic analyses of each parasite isolate.
REFERENCES1. Chai JY. In Fried B, Toledo R eds, Echinostomes in humans. The Biology of Echinostomes. 2009, New York, USA. Springer. pp 147-183.
2. Huffman JE, Fried B. Echinostoma and echinostomiasis. Adv Parasitol 1990;29:215-269. PMID: 2181828.
3. Ando R, Ozaki Y. On four new species of trematodes of the family Echinostomatidae. Dobutsugaku Zasshi 1923;35:108-119 (in Japanese).
4. Shibue H. Studies on the trematodes of birds in Kyushu. Kurume Igakkai Zasshi 1954;17:178-183 (in Japanese).
5. Yokohata Y, Hisashi ABE, Jiang YP, Kamiya M. Gastrointestinal helminth fauna of Japanese moles, Mogera spp. Jpn J Vet Res 1989;37:1-13. PMID: 2709647.
6. Ito M, Itagaki T. Survey on wild rodents for endoparasites in Iwate Prefecture, Japan. J Vet Med Sci 2003;65:1151-1153. PMID: 14600361.
7. Majima M. Echinostoma macrorchis found in a man. Tokyo Iji Shinshi 1927;No. 2552:2260-2263.
8. Okabe N, Okabe K. On a human case infected with three species of trematodes. Nippon Iji Shimpo 1972;No. 2531:46-48 (in Japanese).
9. Fischthal JH, Kuntz RE. Some digenetic trematodes of mammals from Taiwan. Proc Helminthol Soc Wash 1975;42:149-157.
10. Fischthal JH, Kuntz RE. Additional records of digenetic trematodes of mammals from Taiwan. Proc Helminthol Soc Wash 1981;48:71-79.
11. Lo CT. Echinostoma macrorchis: life history, population dynamics of intramoluscan stages, and the first and second intermediate hosts. J Parasitol 1995;81:569-576. PMID: 7623199.
12. Chai JY, Hongvanthong B. A small-scale survey of intestinal helminthic infections among the residents near Pakse, Laos. Korean J Parasitol 1998;36:55-58. PMID: 9529864.
13. Chai JY, Park JH, Han ET, Guk SM, Shin EH, Lin A, Kim JL, Sohn WM, Yong TS, Eom KS, Min DY, Hwang EH, Phommasack B, Insisiengmay B, Rim HJ. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane province in Laos. J Helminthol 2005;79:283-289. PMID: 16153322.
14. Chai JY, Han ET, Guk SM, Shin EH, Sohn WM, Yong TS, Eom KS, Lee KH, Jeong HG, Ryang YS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH, Rim HJ. High prevalence of liver and intestinal fluke infections among residents of Savannakhet province in Laos. Korean J Parasitol 2007;45:213-218. PMID: 17876167.
15. Chai JY, Han ET, Shin EH, Sohn WM, Yong TS, Eom KS, Min DY, Um JY, Park MS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH, Rim HJ. High prevalence of Haplorchis taichui, Prosthodendrium molenkampi, and other helminth infections among people in Khammouane province, Lao PDR. Korean J Parasitol 2009;47:243-247. PMID: 19724697.
16. Chai JY, Yong TS, Yong TS, Eom KS, Min DY, Shin EH, Banouvong V, Insisiengmay B, Insisiengmay S, Phommasack B, Rim HJ. Prevalence of the intestinal flukes Haplorchis taichui and H. yokogawai in a mountainous area of Phongsaly province, Lao PDR. Korean J Parasitol 2010;48:339-342. PMID: 21234239.
17. Chai JY, Sohn WM, Yong TS, Eom KS, Min DY, Hoang EH, Phommasack B, Insisiengmay B, Rim HJ. Echinostome flukes recovered from humans in Khammouane province, Lao PDR. Korean J Parasitol 2012;50:269-272. PMID: 22949759.
18. Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisiengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet province, Lao PDR. Korean J Parasitol 2008;46:253-260. PMID: 19127332.
19. Yamaguti S. Systema helminthum. Vol. I. The digenetic trematodes of vertebrates (Part I & II). 1958, New York, New York, USA. Interscience Publishers Inc.. p 630.
20. Yamaguti S. Studies on the helminth fauna of Japan. Part. 27. Trematodes of mammals, II. Jpn J Med Sci 1939;1:131-151.
21. Yamaguti S. 1971. Genus Echinostoma Rud., 1809. Synopsis of Digenetic Trematodes of Vertebrates (Vol. I & II). 1971, Tokyo, Japan. Keigaku Publishing Co.. pp 529-533.
22. Bashikirova E. In Skrjabin KI ed, Family Echinostomatidae Dietz, 1909. Trematodes of Animals and Man. 1947, Vol. I:Moscow-Leningrad, Russia. Academy of Sciences of the USSR. pp 246-305 (English translated version).
23. Skrjabin KI, Petrov AM, Bashikirova E. In Skrjabin KI ed, Echinostomes of domestic and wild birds in USSR. Trematodes of Animals and Man. 1947, Vol. I:Moscow-Leningrad, Russia. Academy of Sciences of the USSR. pp 306-407 (English translated version).
24. Skrjabin KI, Bashikirova E. In Skrjabin KI ed, Family Echinostomatidae Dietz, 1909. Trematodes of Animals and Man. 1956, Vol. XII:Moscow, Russia. Academy of Sciences of the USSR. pp 53-930.
25. Odening K. Trematoden aus Indischen Vögeln des Berliner Tierparks. Z Parasitenkd 1962;21:381-425. PMID: 14481105.
26. Kanev I, Fried B, Radev V. Collar spine models in the genus Echinostoma (Trematoda: Echinostomatidae). Parasitol Res 2009;105:921-927. PMID: 19468754.
27. Ochi S. Studies on the life cycle of Echinostoma macrorchis. Tokyo Joigaku Zasshi 1931;1:12-32 (in Japanese).
28. Fried B, Irwin SWB, Lorry SF. Scanning electron microscopy of Echinostoma trivolvis and E. caproni (Trematoda) adults from experimental infection in the golden hamster. J Nat Hist 1990;24:433-440.
29. Maldonado A Jr, Loker ES, Morgan JAT, Rey L, Lanfredi RM. Description of the adult worms of a new Brazilian isolate of Echinostoma paraensei (Plathyhelminthes: Digenea) from its natural vertebrate host Nectomys squamipes by light and scanning electron microscopy and molecular analysis. Parasitol Res 2001;87:840-848. PMID: 11688891.
30. McCarthy AM. Scanning electron microscopy of adult Echinoparyphium recurvatum (von Linstow, 1873) (Digenea: Echinostomatidae) from Britain. J Helminthol 2011;85:453-457. PMID: 21208510.
31. Didyk AS, Burt MD.
Himasthla limnodromi n. sp. (Digenea: Echinostomatidae) from the short-billed dowitcher, Limnodromus griseus (Aves: Scolopacidae). J Parasitol 1997;83:1124-1127. PMID: 9406790.
32. Han ET, Han KY, Chai JY. Tegumental ultrastructure of the juvenile and adult Himasthla alincia (Digenea: Echinostomatidae). Korean J Parasitol 2003;41:17-25. PMID: 12666726.
33. Han ET, Whang JD, Chai JY. Himasthla alincia (Echinostomatidae): metacercariae in brackish water bivalves and their growth and development in experimental animals. J Parasitol 2009;95:1415-1420. PMID: 19663537.
34. Han ET, Choi MS, Choi SY, Chai JY. Surface ultrastructure of juvenile and adult Acanthoparyphium tyosenense (Digenea: Echinostomatidae). J Parasitol 2011;97:1049-1054. PMID: 21711106.
35. Ando R, Tsuyuki H. Studies on intestinal parasites taking rats as their final host (II) On Echinostoma in Nagoya city and its second intermediate host. Tokyo Iji Shinshi 1923;No. 2340:1487-1499 (in Japanese).
36. Takahashi S. The life cycles of Echinostoma cinetorchis and E. macrorchis, particularly on their first and second intermediate hosts. Zasshi Fukuoka Ika Daigaku 1927;20:712-723 (in Japanese).
37. Kurisu S. Study on trematodes who take Viviparus as their intermediate host. Seiikai Zasshi 1930;49:65-73 (in Japanese).
38. Kurokawa T. Studies on trematodes taking Bulimus (Parafossarulus) striatus (Pilsbry) as their intermediate host and on their metacercariae. Tokyo Iji Shinshi 1935;No. 2937:1795-1800 (in Japanese).
39. Yamashita J. Echinostome. Progr Med Parasitol Japan 1964;1:289-313.
40. Ishii N. Studies on rat trematodes. Jikken Igaku Zasshi 1935;19:1122-1128 (in Japanese).
41. Komiya Y. Metacercariae in Japan and adjacent territories. Progr Med Parasitol Japan 1965;2:1-328.
Fig. 1The snail host, metacercariae, and adults of Echinostoma macrorchis. (A) Cipangopaludina sp. snails purchased in a market in Vientiane Municipality, Lao PDR. (B) A metacercaria bending within the cyst and showing the characteristic excretory granules. Bar=25 µm. (C) An adult fluke recovered from an experimental rat at day 14 post-infection showing the prominent head collar, 2 large and characteristically shaped testes, and cirrus sac extending beyond the anterior half of the ventral sucker. Bar=0.6 mm. (D) Another adult worm (aged 14 days) showing similar features of the head collar and 2 testes. Bar=0.6 mm. (E) Head collar of an adult fluke showing total 43 collar spines arranged in 2 alternating rows. Bar=50 µm. (F) Head collar of another adult fluke showing total 45 collar spines arranged in 2 alternating rows. Bar=50 µm. 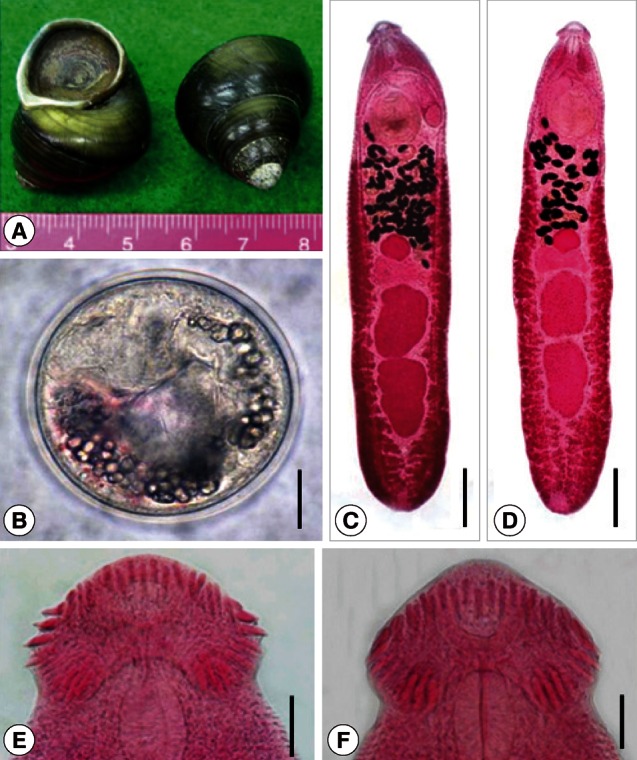
Fig. 2Scanning electron microscopic views of Echinostoma macrorchis adults. (A) Ventral view of a whole worm recovered from a rat at day 14 post-infection. Bar=0.7 mm. (B) A close-up view of its head collar. Bar=50 µm. (C) Dorsal view of the head collar and collar spines showing dorsal alternating spines. Bar=50 µm. (D) Ventral view of another worm stooping its anterior body and showing the dorsal collar spines. Bar=0.7 mm. (E) Close-up view of (A) (near the anterior end of the worm) showing the ventral sucker and a cirrus protruded from the genital pore with several tiny spines near its tip. Bar=80 µm. (F) Tegument of the mid-ventral portion of the body showing scale-like spines. Bar=50 µm. (G) Tegument of the mid-dorsal portion of the body devoid of spines. Bar=50 µm. 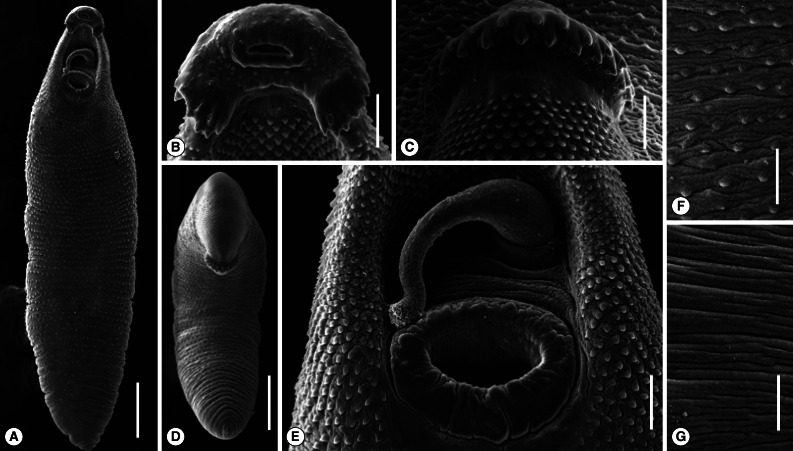
Table 1.Comparison of Echinostoma macrorchis adults recovered from experimental rats and a cat with those described by previous authors
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||