AbstractNeurognathostomiasis is a severe form of human gnathostomiasis which can lead to disease and death. Diagnosis of neurognathostomiasis is made presumptively by using clinical manifestations. Immunoblotting, which recognizes antigenic components of molecular mass 21 kDa and 24 kDa in larval extracts of Gnathostoma spinigerum (Gs 21/24), has high sensitivity and specificity for diagnosis of neurognathostomiasis. However, only very small amounts of the Gs 21/24 antigens can be prepared from parasites harvested from natural or experimental animals. To overcome this problem, we recently produced a recombinant matrix metalloproteinase (rMMP) protein from G. spinigerum. In this study, we evaluated this rMMP alongside the Gs 21/24 antigens for serodiagnosis of human neurognathostomiasis. We studied sera from 40 patients from Srinagarind Hospital, Khon Kaen University, Thailand, with clinical criteria consistent with those of neurognathostomiasis, and sera from 30 healthy control adults from Thailand. All sera were tested for specific IgG antibodies against both G. spinigerum crude larval extract and rMMP protein using immunoblot analysis. The sensitivity and specificity for both antigenic preparations were all 100%. These results show that G. spinigerum rMMP protein can be used as an alternative diagnostic antigen, in place of larval extract, for serodiagnosis of neurognathostomiasis.
Human gnathostomiasis is an important food-borne helminthic zoonosis caused by spirurid nematodes of the genus Gnathostoma and is endemic in Asia and the Americas [1-3]. Generally gnathostomiasis is not a life-threatening disease, fatalities being recorded only occasionally among neurognathostomiasis patients [2,4]. Gnathostoma spinigerum has been reported as the only causative agent for neurognathostomiasis in Southeast Asia, particularly Thailand, the only region where this condition exists [4]. Human infection occurs by consuming raw or inadequately cooked foods, e.g. freshwater fishes, frogs, and chickens, which harbor Gnathostoma advanced third stage larvae (AL3). The larvae migrate to the CNS to cause radiculomyelitis or radiculoencephalomyelitis. Subarachnoid hemorrhage (SAH) can occur, sometimes leading to death [5-7]. Definitive diagnosis of helminthic CNS infections is difficult. The chances of recovering worm specimens are extremely low [4]. The most reliable diagnostic tool is a serologic test, such as immunoblotting using Gnathostoma AL3 extract that is known to contain 2 antigenic peptides with approximate molecular masses of 21 kDa and 24 kDa [8]. However, only tiny quantities of the specific antigens can be obtained from AL3, and laboratory maintenance of the life-cycle to ensure supply is expensive and time consuming. Therefore, we evaluated the diagnostic use of a recombinant matrix metalloproteinase (rMMP) protein [9] for immunodiagnosis of neurognathostomiasis. Recombinant proteins can be produced in large quantities whenever required.
This study prospectively enrolled 40 patients who were suspected of having neurognathostomiasis at the Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand, between 2003 and 2012. A presumptive diagnosis of neurognathostomiasis was made using clinical criteria following a previous report [8]. The major criteria were eating raw food possibly contaminated with living Gnathostoma infective stage larvae from an endemic area, and acute-onset neurologic symptoms. Minor criteria were migratory swelling, radicular pain, blood eosinophilia > 500 cells/mm3, eosinophils in cerebrospinal fluid, and suggestive results by neuroimaging [7,10,11]. A presumptive diagnosis of neurognathostomiasis was made when a person satisfied both of the major criteria and 1 of the minor criteria. All patients were positive for the 21 kDa and/or 24 kDa antigenic components of the G. spinigerum larval extract (Gs 21/24) but yielded negative serologic results for angiostrongyliasis [12], paragonimiasis [13], and fascioliasis [14] in immunoblotting, and also negative for cysticercosis [15] by ELISA. The negative control group included 30 samples from healthy adult volunteers who at the time of blood collection were found to be free from any intestinal parasite after stool examination using the formalin ethyl acetate concentration technique [16]. Informed consent was obtained from all human adult participants. The study protocol was approved by the Khon Kaen University Ethics Committee for Human Research (HE541293).
The rMMP protein was constructed from a cDNA encoding MMP protein of G. spinigerum larvae, cloned and expressed in Escherichia coli [9]. It had a molecular mass of approximately 102 kDa with the fusion-tagged protein. The G. spinigerum larval extract [17] and purified fusion-tagged rMMP protein [9] were separately characterized by SDS-PAGE followed by immunoblotting as previously described [17] with some modifications. The membranes blotted with G. spinigerum larval extract and rMMP were incubated in 1% skimmed milk in PBS, pH 7.5, containing 0.1% Tween-20 (PBST) for 30 min for blocking non-specific binding sites, washed, and then the membrane was cut into ~3 mm wide strips (9.8 µg protein/strip) for both antigens. Each strip was incubated with 1:100 diluted individual human serum sample (in 1% skimmed milk in PBS, pH 7.5) for 2 hr at room temperature. For immunoblotting against the rMMP protein, each serum sample was absorbed with E. coli lysate before use. The membranes were washed with 1% skimmed milk in PBST (5 times each) and incubated with goat anti-human IgG (H+L) HRP conjugate (Invitrogen) at a dilution of 1:4,000 in 1% skimmed milk in PBST, for 2 hr at room temperature. After 5 washes with 1% skimmed milk in PBST, the strips were developed with 3,3'-diaminobenzidine tetrahydrochloride and H2O2 as substrate, and the reaction stopped with distilled water. The diagnostic parameters of sensitivity and specificity were calculated as previously described [18].
Immunoblot analysis employing the G. spinigerum larval extract of 21 kDa and 24 kDa antigenic components (Gs 21/24) (Fig. 1A) and the purified fusion-tagged rMMP protein (Fig. 1B) was evaluated using individual sera from healthy controls and from neurognathostomiasis patients. All serum samples from the neurognathostomiasis patients strongly reacted with both antigens. None of the 30 healthy control sera showed positive seroreactivity. The calculated diagnostic sensitivity and specificity were therefore 100% in each case.
Definitive diagnosis of neurognathostomiasis requires invasive procedures for demonstration of parasites [4]. The procedures need experienced personnel and laboratory support, and cannot be performed in general practice. Practically, diagnosis is made by clinical features, a history of eating parasite-contaminated foods, blood eosinophilia, eosinophils in cerebrospinal fluid, suggestive neuroimaging results, and serological findings especially via immunoblotting using the Gs 21/24 antigens [8]. Our results showed that the rMMP is as good as the Gs 21/24 antigens for serologic diagnosis of human neurognathostomiasis.
As neurognathostomiasis has been reported in Asia, i.e., Thailand, Laos, Japan, Mayanmar, and South Korea, as well as in European travelers returning from endemic areas [4], the mass production of G. spinigerum rMMP protein creates the potential for development of a serodiagnostic kit, i.e., immunochromatographic test. The method can be used not only for neurognathostomiasis but also for other forms of gnathostomiasis. The supportive diagnosis will help clinicians for prompt treatment of this harmful disease. Importantly, this diagnostic tool will be helpful for neurosurgeons considering management of intracranial hemorrhage patients without conventional invasive therapy.
Strategic Scholarships for Frontier Research Networks for Ph.D. program Thai Doctoral degree (CHE Ph.D. Scholarship)I 56320
the Faculty of Medicine, Khon Kaen University, Khon Kaen, ThailandI 56320 TRF Senior Research Scholar GrantRTA5580004 Association for Preventive Medicine of Japan ACKNOWLEDGMENTSThis research was supported by grants from the Office of the Higher Education Commission for supporting for grant funded under the Strategic Scholarships for Frontier Research Networks for Ph.D. program Thai Doctoral degree (CHE Ph.D. Scholarship), and the Faculty of Medicine, Khon Kaen University University, Khon Kaen, Thailand grant no. I 56320. Penchom Janwan was supported by a CHE Ph.D. Scholarship. Wanchai Maleewong and Pewpan M. Intapan were supported by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004. This research was also funded by a grant from the Association for Preventive Medicine of Japan in 2011 and 2012 to Wanchai Maleewong and Hiroshi Yamasaki.
REFERENCES1. Miyazaki I. On the genus Gnathostoma and human gnathostomiasis, with special reference to Japan. Exp Parasitol 1960;9:338-370. PMID: 14423082.
2. Daengsvang S. Gnathostomiasis in Southeast Asia. Southeast Asian J Trop Med Public Health 1981;12:319-332. PMID: 7342319.
3. León-Règagnon V, Osorio-Sarabia D, García-Prieto L, Akahane H, Lamothe-Argumedo R, Koga M, Messina-Robles M, Alvarez-Guerrero C. Study of the etiological agent of gnathostomosis in Nayarit, Mexico. Parasitol Int 2002;51:201-204. PMID: 12113759.
4. Katchanov J, Sawanyawisuth K, Chotmongkoi V, Nawa Y. Neurognathostomiasis, a neglected parasitosis of the central nervous system. Emerg Infect Dis 2011;17:1174-1180. PMID: 21762569.
5. Chitanondh H, Rosen L. Fatal eosinophilic encephalomyelitis caused by the nematode Gnathostoma spinigerum. Am J Trop Med Hyg 1967;16:638-645. PMID: 6053531.
6. Punyagupta S, Juttijudata P, Bunnag T, Comer DS. Two fatal cases of eosinophilic myeloencephalitis a newly recognized disease caused by Gnathostoma spinigerum. Trans R Soc Trop Med Hyg 1968;62:801-809. PMID: 5729570.
7. Boongird P, Phuapradit P, Siridej N, Chirachariyavej T, Chuahirun S, Vejjajiva A. Neurological manifestations of gnathostomiasis. J Neurol Sci 1977;31:279-291. PMID: 839236.
8. Intapan PM, Khotsri P, Kanpittaya J, Chotmongkol V, Sawanyawisuth K, Maleewong W. Immunoblot diagnostic test for neurognathostomiasis. Am J Trop Med Hyg 2010;83:927-929. PMID: 20889894.
9. Janwan P, Intapan PM, Yamasaki H, Laummaunwai P, Sawanyawisuth K, Wongkham C, Tayapiwatana C, Kitkhuandee A, Lulitanond V, Nawa Y, Maleewong W. Application of recombinant Gnathostoma spinigerum matrix metalloproteinase-like protein for serodiagnosis of human gnathostomiasis by immunoblotting. Am J Trop Med Hyg 2013;89:63-67. PMID: 23716413.
10. Sawanyawisuth K, Tiamkao S, Kanpittaya J, Dekumyoy P, Jitpimolmard S. MR imaging findings in cerebrospinal gnathostomiasis. AJNR Am J Neuroradiol 2004;25:446-449. PMID: 15037471.
11. Kanpittaya J, Sawanyawisuth K, Intapan PM, Khotsri P, Chotmongkol V, Maleewong W. A comparative study of neuroimaging features between human neuro-gnathostomiasis and angiostrongyliasis. Neurol Sci 2012;33:893-898. PMID: 22124854.
12. Maleewong W, Sombatsawat P, Intapan PM, Wongkham C, Chotmongkol V. Immunoblot evaluation of the specificity of the 29-kDa antigen from young adult female worms Angiostrongylus cantonensis for immunodiagnosis of human angiostrongyliasis. Asian Pac J Allergy Immunol 2001;19:267-273. PMID: 12009076.
13. Maleewong W, Wongkham C, Pariyanonda S, Intapan PM. Analysis of antibody levels before and after praziquantel treatment in human paragonimiasis heterotremus. Asian Pac J Allergy Immunol 1992;10:69-72. PMID: 1418188.
14. Intapan PM, Maleewong W, Wongkham C, Tomanakarn K, Ieamviteevanich K, Pipitgool V, Sukolapong V. Excretory-secretory antigenic components of adult Fasciola gigantica recognized by infected human sera. Southeast Asian J Trop Med Public Health 1998;29:579-583. PMID: 10437961.
15. Intapan PM, Khotsri P, Kanpittaya J, Chotmongkol V, Maleewong W, Morakote N. Evaluation of IgG4 and total IgG antibodies against cysticerci and peptide antigens for the diagnosis of human neurocysticercosis by ELISA. Asian Pac J Allergy Immunol 2008;26:237-244. PMID: 19317343.
16. Elkins DB, Haswell-Elkins M, Anderson RM. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans R Soc Trop Med Hyg 1986;80:774-792. PMID: 3603617.
17. Laummaunwai P, Sawanyawisuth K, Intapan PM, Chotmongkol V, Wongkham C, Maleewong W. Evaluation of human IgG class and subclass antibodies to a 24 kDa antigenic component of Gnathostoma spinigerum for the serodiagnosis of gnathostomiasis. Parasitol Res 2007;101:703-708. PMID: 17468971.
18. Galen RS. Predictive value and efficiency of laboratory testing. Pediatr Clin North Am 1980;27:861-869. PMID: 7454413.
Fig. 1Representative immunoblotting patterns (A, B) when human sera were tested against the Gnathostoma spinigerum larval extract containing 21 kDa and 24 kDa antigenic components (Gs 21/24) (A), and when the same sera were tested against the recombinant matrix metalloproteinase (rMMP) protein (B). Antigens were probed with the sera from pooled negative controls (N), pooled human gnathostomiasis patients (P), clinically confirmed neurognathostomiasis patients (1-10) and healthy controls (11-15). The arrows indicate the specific immunoreactive bands at approximately 21 kDa and 24 kDa (Gs 21/24) and at approximately 102 kDa (rMMP protein with the fusion-tagged protein). 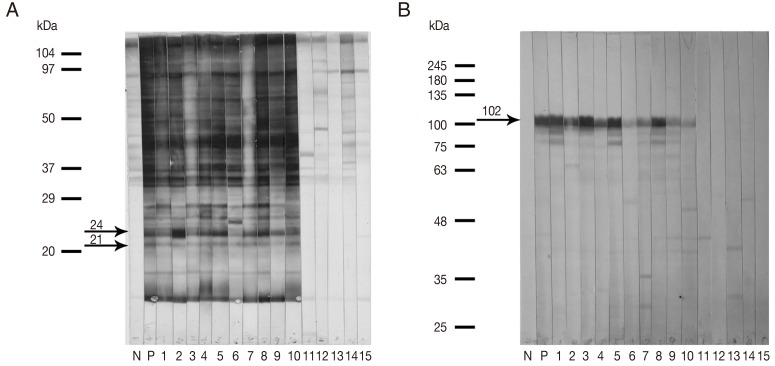
|
|
|||||||||||||||||||||||||||||||||||||