INTRODUCTION
Epidemiological surveillance data shows an estimated 2.6 million (2.3-2.8 million) people are newly infected with HIV and is also believed that an average of 1.8 million (1.6-2.1 million) people die annually because of AIDS worldwide. Intestinal parasitic infections are one of the most frequent causes of morbidity and mortality in HIV-infected patients (UNAIDS). A consistent association between HIV infection and intestinal parasitosis has been reported in tropical regions [1] and more so in patients with CD4+ T-cell counts less than 200 cells/mm3 [2]. Data on correlation of HIV/AIDS with enteric parasitic infection in South Asian region have reported association of several species of pathogenic protozoa such as Cryptosporidium spp., Cystoisospora belli, Cyclospora cayetanensis, Giardia lamblia, Entamoeba histolytica, Blastocystis hominis, and microsporidia species [3]. The clinical spectrum caused by intestinal parasites among HIV positive patients ranges from asymptomatic to severe infections leading to chronic diarrhea, dehydration, and malabsorption [4]. It has also been observed that antiretroviral therapy (ART) can restore immunity by increasing both memory and naive CD4+ cells against many opportunistic pathogens [5,6]. The use of ART has dramatically reduced the incidence of diarrhea due to Cryptosporidium spp., C. belli, and microsporidia primarily due to immune reconstitution after ART initiation. In the absence of a vaccine and affordable ART in many parts of the world, people living with HIV/AIDS, especially in developing countries, remain at considerable risk for various opportunistic infections [6]. In this context, the present study was undertaken with an objective of assessing the presence of intestinal parasitic infections in HIV seropositive patients and its correlation with CD4+ T-cell count in India.
MATERIALS AND METHODS
The study was conducted from June 2010 to June 2013 and participating individuals were enrolled from both in-patient and out-patient departments of our tertiary health care centre, New Delhi, India. Stool samples were obtained both from HIV seropositive patients on ART termed as “on ART HIV seropositives with diarrhea (n=100)” and “ART naive HIV seropositive patients without diarrhea (n=100)” (Table 1). Both of these groups of patients were enrolled as “cases”. The “on-ART HIV seropositive patients with diarrhea” included 45 patients with CD4+ T-cell count less than 200 cells/µl and 55 patients with CD4+ T-cell count between 200-350 cells/µl. Whereas all “ART naive HIV seropositive patients without diarrhea (n=100) had CD4+ T-cell count of more than 350 cells/µl. In addition to above 2 groups, 200 HIV uninfected patients comprising of 100 patients with diarrhea and 100 patients without diarrhea were taken as “controls”. Diarrhea was defined as the passage of liquid or unformed stool at an increased frequency according to WHO in 2009 (http://www.who.int/topics/diarrhoea/en/) and was further categorized as ‘acute’, ‘persistent’, and ‘chronic’ if lasts for duration of 7 days, 8-15 days, and for more than 15 days, respectively. In the present study, patients with complaints of persistent and chronic diarrhea were put together as chronic diarrhea.
For microscopic examinations, stool sample processing was carried out depending on the consistency of the samples received. In case of watery stools, direct wet-mounts (both in normal saline and 3% Lugol’s iodine) were prepared and a fecal smear was made. For the formed stools, formol-ether concentration technique was used [7] and the sediment was used for preparation of wet-mount and fecal smears. Modified Ziehl-Neelsen (MAF) staining was performed on fecal smears to detect oocysts of Cryptosporidium spp., C. cayetanensisi, and C. belli [8] and modified trichrome staining for the detection of microsporidia species [9].
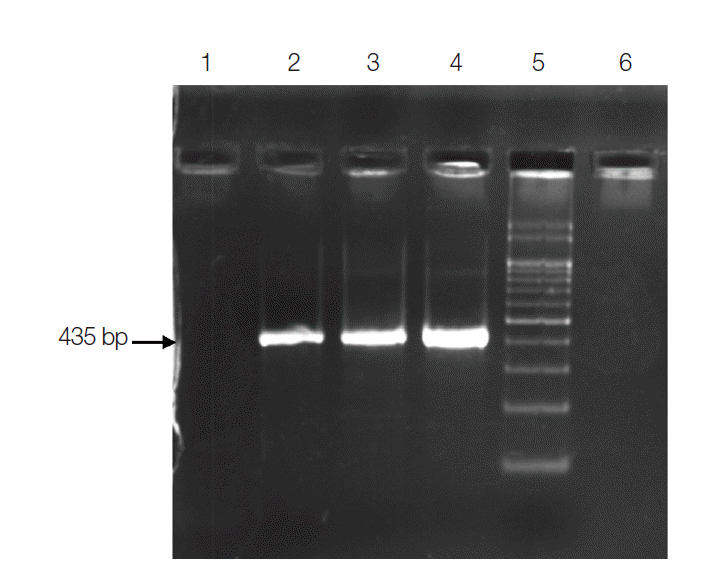
Diagnostic PCR assay was carried out targeting 18S rRNA gene of the genus Cryptosporidium [10,11], C. cayetanensisi [11], C. belli [12], and Enterocytozoon bieneusi (E. bieneusi) [13] using published primers. Amplified PCR products were visualized in 2% gels stained with ethidium bromide. PCR assay for the genus Cryptosporidium was optimized using an equal mixture of forward primer CPB-DIAGF (5´-AGCTCGTAGTTGGATTTCTG-3´) and reverse primer CPB-DIAGR (5´-TAAGGTGCTGAAGGAGTAAGG-3´) in a volume of 25 µl. PCR assay was performed using an initial denaturation temperature of 94˚C for 5 min followed by 35 cycles of denaturation at 94˚C for 30 sec. Annealing was done at 56˚C for 30 sec followed by extension at 72˚C for 1 min. Final extension was given for 10 min at 72˚C. The amplified PCR product was of size 435 bp.
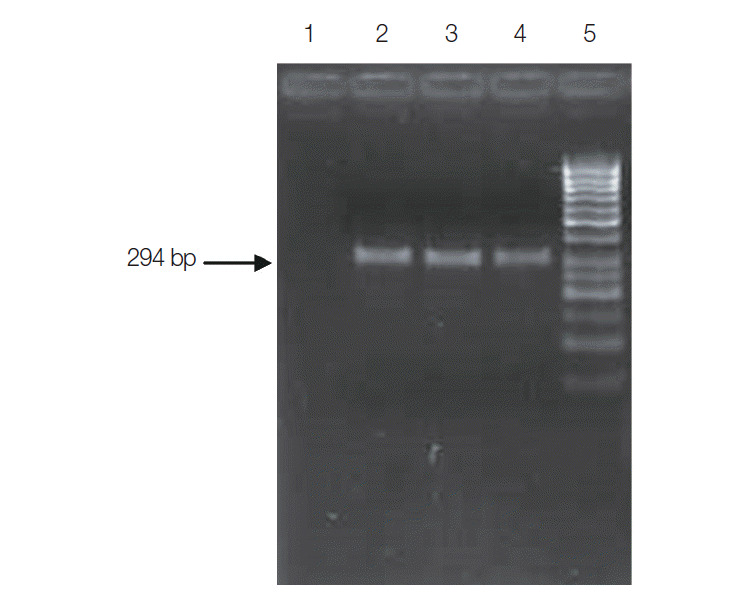
PCR assay for the detection of C. cayetanensisi was optimized using nested PCR assay. Oligonucleotide primer F1E (5´-TACCCAATGAAAACAGTT-3´) was used as the forward primer and R2B (5´-CAGGAGAAGCCAAGGTAG-3´) as the reverse primer for the external round of PCR assay. The external round of PCR assay amplified a primary amplicon of 636 bp, whereas the set of internal primer pair F3E (5´-CCTTCCGCGCTTCGCTGCGT-3´) and R4B (5´-CGTCTTCAAACCCCCTACTG-3´) amplified a 294 bp region. The PCR assay procedure consisted of initial denaturation at 94˚C for 5 min followed by 35 cycles of denaturation at 94˚C for 30 sec, annealing at 53˚C for 30 sec, extension at 72˚C for 90 sec, and a final extension at 72˚C for 10 min. PCR cycling condition for the nested round was the same as the external round except for change in annealing temperature to 61˚C.
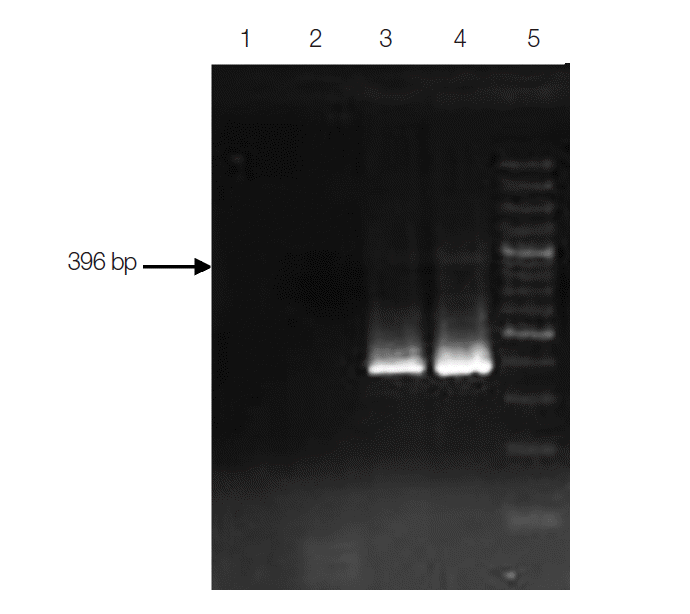
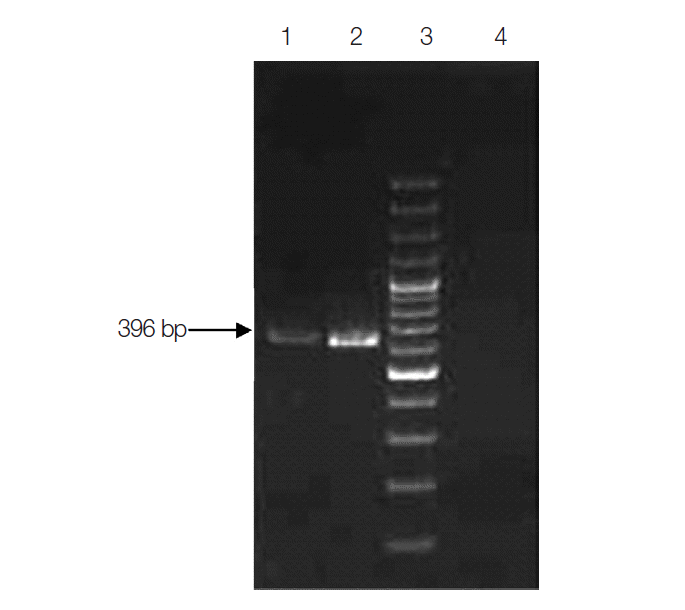
PCR assay for the detection of C. belli was optimized as a nested PCR assay. The external round PCR assay was carried out using IsoFO (5´-GTGCCTCTTCCTCTGGAAGG-3´) as the forward primer and IsoRO (5´-GCACTCCACCCAGTTAAGTGC-3´) as the reverse primer. External round amplified a PCR product of 559 bp. IsoFI (5´-CGATGGATCATTCAAGTTTC-3´) and IsoRI (5´-ACCACGTACACACCCCTAAG-3´) were used as forward and reverse primers for the internal round. Internal round amplified a PCR product of size 396 bp. The PCR protocol consisted of initial denaturation at 94˚C for 3 min followed by 35 cycles of denaturation at 94˚C for 1 min, annealing at 61˚C for 2 min, extension at 72˚C for 3 min, and a final extension at 72˚C for 10 min. PCR cycling condition for the nested round was the same as the external round except for changes in annealing temperature to 57˚C.
PCR assay for exclusive detection of E. bieneusi was optimized as a single round PCR assay using an equal mixture of forward primers EBIEF1 (5´-GAAACTTGTCCACTCCTTACG-3´) and reverse primer EBIER1 (5´-CCATGCACCACTCCTGCCATT-3´) amplifying a region of 607 bp. The PCR protocol consisted of initial denaturation at 94˚C for 5 min followed by 35 cycles of denaturation at 94˚C for 30 sec, annealing at 56˚C for 30 sec, extension at 72˚C for 90 sec, and a final extension at 72˚C for 10 min.
Ethical approval was obtained from the institutional ethics committee before the commencement of the study. All participants were informed that the procedure used did not pose any potential risk and their identities, and personal particulars will be kept confidential. During the meetings, enrolled individuals were also informed that their participation is absolutely voluntary, and they can withdraw from the study at any given point of time without explaining any reasons.
Analyses were done using STATA software, version 11 (Stata-Corp. College Station, Texas, USA). Descriptive statistics were carried out. Odds ratio (OR) and 95% confidence intervals (CI) were calculated by using a bi-variable logistic regression. Tests were considered significant at P-value<0.05.
RESULTS
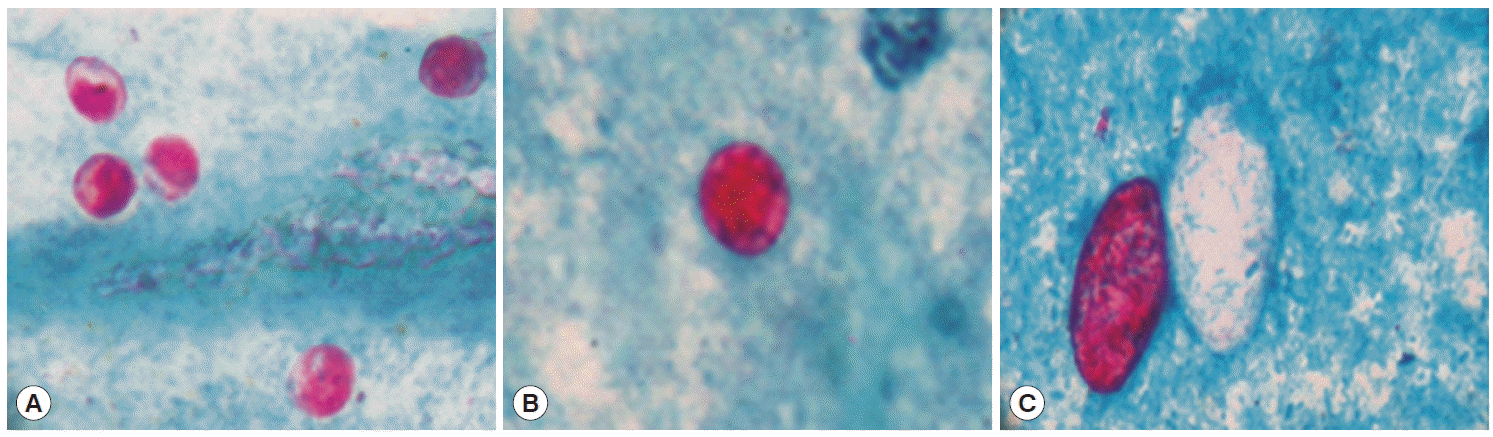
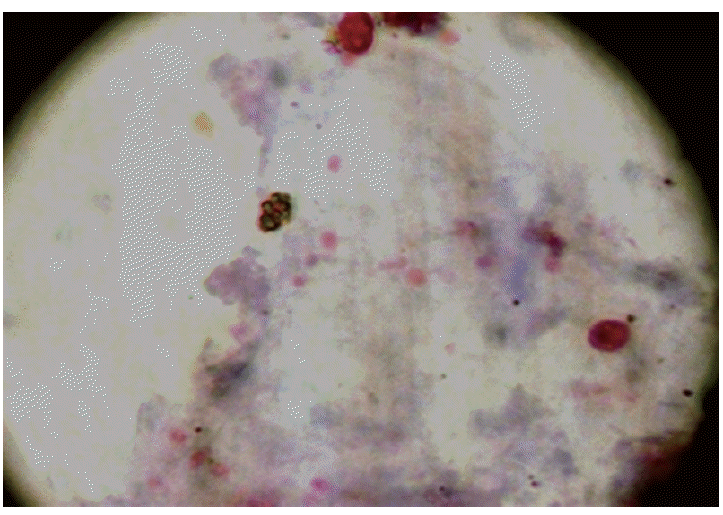
The median age for the cases (n=200) and controls (n=200) was 36 years (range 19-59) and 39 years (19-65), respectively (Table 1). Morphologically, a total of 123 out of 400 patients were positive for any kinds of intestinal parasites. The cumulative number of positive patients was 145 because of multiple infections. The parasite species included Cryptosporidium spp. (n=13), C. cayetanensisi (n=3), C. belli (n=15), Strongyloides stercoralis (n=5), G. lamblia (n=32), E. histolytica/E. dispar (n=15), Ascaris lumbricoides (n=2), Hymenolepis nana (n=10), Entamoeba coli (n=11), Endolimax nana (n=9), and B. hominis (n=30). The environmentally resistant oocysts of Cryptosporidium spp. and C. cayetanensisi measured 4-6 µm and 8- 10 µm, respectively (Fig. 1A, B), whereas, oocysts of C. belli were oval and measured 20-30×10-15 µm in size (Fig. 1C). Spores of microsporidia appeared as small pink dots measuring 1-2 µm in size (Fig. 2). All the measurements were done using the standard micrometry technique.
Subjecting all the clinical specimens to PCR assays, 2 additional cases of Cryptosporidium and 5 cases of E. bieneusi were detected. Hence, a total of 130 (32.5%) out of 400 patients were positive for any kinds of intestinal parasites using both microscopic examinations and PCR assay. The cumulative number of positive patients was 152. Of these, 43 (28%) were infected with opportunistic, 59 (39%) were with non-opportunistic pathogenic, and 50 (33%) were with non-pathogenic parasites (Table 2). PCR product of sizes 435 bp, 294 bp, 396 bp, and 607 bp were obtained for Cryptosporidium spp., C. cayetanensisi, C. belli, and E. bieneusi, respectively (Figs. 3-6).
“On-ART HIV seropositive patients with diarrhea” (n = 100)
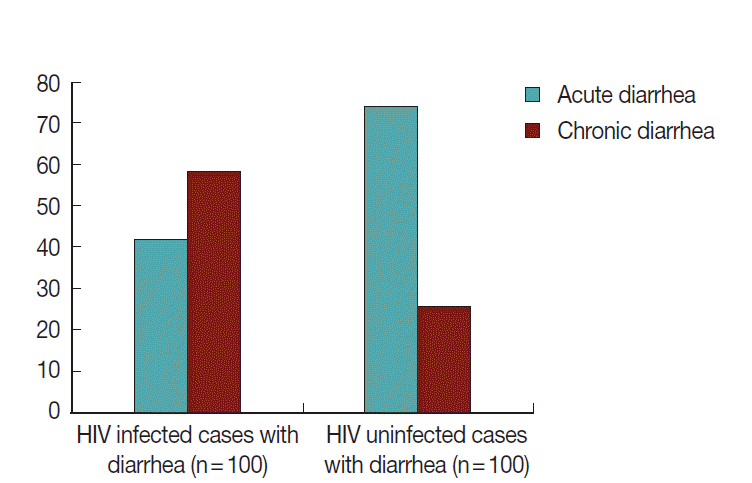
In this group, 42% (42/100) had acute and 58% (58/100) had chronic diarrhea (Fig. 7). Of 100 patients, 49 (49.0%) were positive for any kinds of parasites. The cumulative number of parasite positive patients was 70 because of multiple parasite infections, and among them 33 (47%) were opportunistic parasite positives, 21 (30%) were non-opportunistic pathogenic parasite positives, and 16 (23%) were non-pathogenic parasite positives (Table 3). Of these 70 patients, 23 (33%) and 47 (67%) were having acute and chronic diarrhea, respectively (Fig. 8). Although the difference between these 2 was 2-fold, it was statistically not significant.
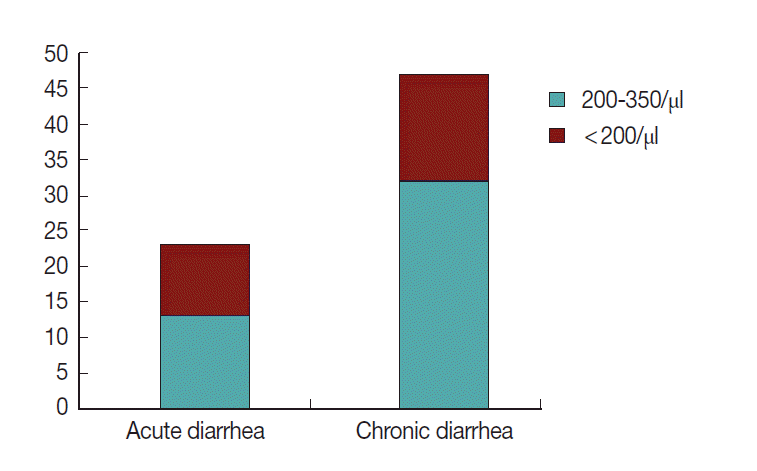
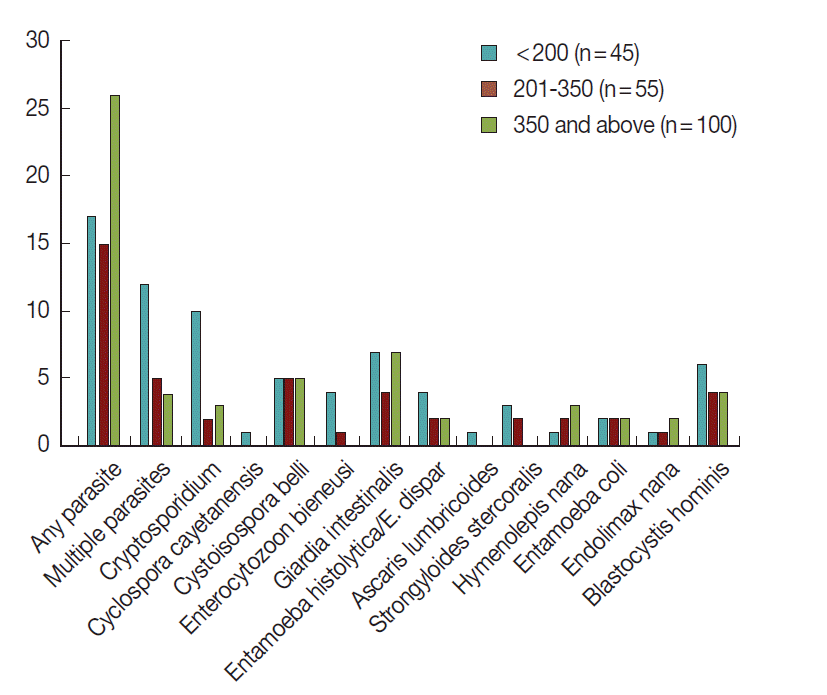
Of the 49 HIV seropositive patients with diarrhea on ART who had parasitic infections, 29 (59.2%) were patients with CD4+ T-cell count less than 200 cells/µl and 20 (40.8%) were with CD4+ T-cell count between 200-350 cells/µl (Fig. 9). Increased frequencies of multiple parasites were observed in individuals with CD4+ T-cell count less than 200 cells/µl (OR=3.63; 95% CI, 1.17-11.28; P=0.02). In addition, higher frequency of cryptosporidiosis was detected in individuals with CD4+ T-cell count less than 200 cells/µl (OR=8.5; 95% CI, 2.75-26.66).
“ART naive HIV seropositive patients without diarrhea” (n = 100)
In this group, 27 (27.0%) of 100 patients were positive for any kinds of parasites. The cumulative number of parasite infections was 28. These 28 intestinal parasites included 8 (29%) opportunistic parasites, 12 (42%) non-opportunistic parasites, and 8 (29%) non-pathogenic parasites (Table 3). These patients mostly presented with single parasitic infections, except 1 who showed multiple parasitic infections (Fig. 9).
HIV uninfected patients with or without diarrhea (n = 100 + 100)
In this group, 40 (40.0%) of 100 patients with diarrhea and 14 (14.0%) of 100 patients without diarrhea were positive for any kinds of parasites. Amongst the individuals with diarrhea, 74% had acute and 26% had chronic diarrhea (Fig. 7). Two (5.0%) of 40 cases were positive for C. cayetanensisi (opportunistic parasites), and 20 (50%) were with non-opportunistic intestinal parasites. The remaining 18 (45%) were with non-pathogenic parasites. However, in HIV uninfected patients without diarrhea, 6 (42.0%) of the 14 cases were with non-opportunistic parasites, and 8 (58%) were with non-pathogenic parasites (Table 3). There were no multiple infections in these groups.
Parasites significantly associated with HIV patients
It was noticeable that Cryptosporidium spp. and C. belli were significantly associated with HIV seropositive patients compared to HIV uninfected (Table 2). When the spectrum of intestinal parasites was compared between “on-ART HIV seropositives with diarrhea” (n=100) and “HIV uninfected patients with diarrhea” (n=100), it was observed that Cryptosporidium spp. and C. belli were significantly associated with HIV seropositive patients (P <0.05) (Table 3). However, no significant association of parasitic infections was observed between ART naive HIV seropositives without diarrhea (n=100) and HIV uninfected patients (non HIV) without diarrhea.
DISCUSSION
Deterioration of immune system caused by HIV makes an individual extremely susceptible to numerous opportunistic infections. Almost 80% of the AIDS patients die because of AIDS related opportunistic infections including intestinal parasitic infections rather than disease per se [14]. The present study primarily intended to determine opportunistic, non-opportunistic, and non-pathogenic intestinal parasites in both diarrheic and non-diarrheic HIV seropositive patients.
To circumvent the limitations of conventional microscopic detection methods, all our clinical specimens were subjected to PCR assays as such assays can detect the presence of a single oocyst in the clinical specimens [15]. Intestinal parasites that were detected in our study population (n=400) included Cryptosporidium spp. (3.8%), C. cayetanensisi (0.8%), C. belli (3.8%), E. bieneusi (1.3), G. intestinalis (8.0%), E. histolytica/E. dispar (3.8%), A. lumbricoides (0.5%), S. stercoralis (1.3%), H. nana (2.5%), E. coli (2.8%), E. nana (2.3%), and B. hominis (7.5%).
A total of 36 HIV seropositive patients were infected with coccidia and/or microsporidia implicating a clear association of these parasites with HIV seropositive patients. Similar reports are available both from developed and developing countries [16-18]. In a study conducted on HIV seropositive patients in Laos Peoples’ Democratic Republic, different intestinal parasites were identified in 78.8% of the patients; however, classical opportunistic pathogens (Cryptosporidium, C. belli, C. cayetanensisi, and microsporidia) were detected at a much lower frequency (6.6%, 4.4%, 2.2%, and 2.9%, respectively) [19]. Cryptosporidium as a cause of diarrhea in HIV seropositive patients has been reported from India [20], and more importantly in patients with CD4+ T-cell counts of less than 200 cells/µl [21]. In agreement with the above mentioned studies and others from developing countries [22-24], it can be attributed that increased susceptibility of HIV seropositive patients to acquire particular parasitic infections is associated with CD4+ T-cell count often at less than 200 cells/µl. At a higher CD4+ T-cell count, generally spontaneous clearing of the parasites take place. In a resource poor setting like ours, patients usually are unaware of the disease and go undiagnosed for long periods and seek for medical consultation much later in the course of the disease. Consequently, patients usually present with profound, persistent, and multiple parasitic infections. Poor personal hygiene, low socioeconomic status, and contaminated drinking water are the additional factors responsible for high frequency of cryptosporidiosis [25].
In our study, Cryptosporidium spp. were identified as the predominant etiology of diarrhea in ‘on-ART’ patients, and this can probably be explained by the fact that these patients were diarrheic and had low CD4+ T-cell counts. Another factor that brings to attention is the adherence to the treatment regimen. The effectiveness of ART highly depends on good compliance of the treatment [26,27]; however; low level of adherence to the treatment is a common observation in low income countries. Similar findings have been observed from Ethiopia, where 28.4% of cryptosporidiosis cases and 10% of cystoisosporiasis cases were reported in patients who were receiving ART. A report from France indicated increase of cystoisosporiasis from 0.4 per 1,000 patients in the pre-ART era (1995-1996) to 4.4 per 1,000 patients in the ART era [28].
Immunosuppression, a consequence of HIV infection favours the occurrence of multiple opportunistic infections [30]. Multiple parasitic infections have been reported at a frequency of 4.8% [31] from Africa and 27.2% [32] from Malaysia. Increased frequency of multiple parasitic infections among HIV seropositive individuals with lower CD4+ T-cell counts probably allows non interfering co-habitation of these parasites in the infected hosts.
Amongst the HIV seropositive patients with diarrhea, 58% had chronic diarrhea and 42% had acute diarrhea. Diarrhea is an important clinical problem among HIV seropositive individuals and is associated with significant impairments in health and quality of life [29]. The present study shows that diarrhea is a concern among the participants regardless of their HIV status though it more likely takes chronic course among HIV infected patients than HIV uninfected group.
Increased frequency of intestinal parasitic infections in the present study population certainly warrants necessary intervention. Routine screening of parasitic infections should be made mandatory especially for all asymptomatic HIV seropositive individuals to administer early and specific treatment.