INTRODUCTION
Acanthamoeba is the causative agent of granulomatous amoebic encephalitis and amoebic keratitis [1]. To transform into the resistant cyst stage of Acanthamoeba, the expression levels of a lot of genes mediating the encystation process have to be regulated during encystation. The comparison of transcriptome between cysts and trophozoites in Acanthamoeba castellanii using cDNA microarray demonstrated that 701 genes were highly expressed more than 2-folds in cysts, and 859 genes were expressed lower in cysts than in trophozoites [2]. However, the regulation mechanisms of the encystation mediating genes in Acanthamoeba are still unknown. Therefore, here we focused on epigenetic-based regulation of gene expression during encystation of Acanthamoeba.
Epigenetic regulation of gene expression is associated with DNA methylation, histone modification, and nucleosome positioning [3]. Histone modification, such as methylation, acetylation, and phosphorylation, affects the state of chromatin and gene expression [4]. Arginine methylation is a post-translational modification occurring in several cytoplasmic and nuclear proteins that modulates chromatin modification, RNA processing, DNA repair, organelle biogenesis, and signaling [5,6].
Protein arginine methyltransferases (PRMTs) transfer the methyl group from S-adenosylmethionine to arginine residues of proteins, and generate monomethyl arginine, symmetric dimethylarginine, or asymmetric dimethylarginine [7]. In humans, 9 PRMTs (PRMT1 to PRMT9) have been identified, and they are classified in 4 types (I to IV) according to the modification that they generate [6]. PRMT1 is the predominant member of the PRMT family [8]. PRMT1 is a type I catalytic enzyme, and it is a histone methyltransferase that methylates arginine 3 on histone H4 which plays a role in activation of gene transcription [9,10]. PRMT1 is also a coactivator of some nuclear receptors as well as transcription factors [11]. Coactivator-associated arginine methyltransferase 1 (CARM1) belongs to a family of previously identified PRMTs, and CARM1 can methylate arginine 17 and 26 of histone H3 [12].
In parasitic protozoa, PRMT1 has been identified in Plasmodium berghei (GenBank accession no. LK023125), Plasmodium falciparum (no. SBT78822), and Entamoeba histolytica (no. KB823531). However, the functions of PRMT1 in parasitic protozoa remain unknown. PRMT1 of P. falciparum methylated only arginine 3 of histone H4 [13], and PRMT1 of E. histolytica dimethylated arginine 8 of histone H4 [14].
In this study, we report the identification and characterization of the PRMT1 homologue in A. castellanii (AcPRMT1) (no. KT345168). For this purpose, we cloned and expressed the protein PRMT1 from our existing cDNA library of A. castellanii and investigated its role in encystation of Acanthamoeba.
MATERIALS AND METHODS
Cultivation of Acanthamoeba trophozoites and cysts
A. castellanii Castellanii (ATCC 30011) was obtained from the American Type Culture Collection. Trophozoites were axenically cultured in PYG media, consisting of proteose peptone 2% (w/v), yeast extract 0.1% (w/v), and glucose 100 mM (w/v), at 25°C in a Sanyo incubator (San Diego, California, USA). Cysts were induced in encystation media (0.1 M KCl, 0.008 M MgSO4, 0.0004 M CaCl2, and 0.02 M 2-amino-2-methyl-1,3-propanediol, pH 9.0) for 3 days [15]. Mature cysts were counted under a light microscope after treating them with 0.5% SDS, and encystation ratios were calculated [16].
Gene expression analysis
Total RNAs from A. castellanii trophozoites and cysts were purified using RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA synthesis was conducted using First Strand cDNA Synthesis Kit (Sigma-Aldrich, Fluka, Zwijndrecht, Netherlands) according to the manufacturer’s recommendations. Real-time PCR was carried out using 20 ng of cDNA as template and specific primers corresponding to the AcPRMT1 and AcPRMT5 genes (Table 1). It was performed using the ABI PRISM® 7000 sequence detection system (Applied Biosystems, Foster City, California, USA), and all reaction mixtures used SYBR Premix Ex Taq (Takara, Otsu, Shiga, Japan). The 18S ribosomal DNA was used as the reference gene [17]. Real-time PCR was performed to determine relative gene expression using the 2ΔΔCT method [18], and experiments were performed in triplicate.
Stable transfection and intracellular localization of PRMT1
To investigate the intracellular localization of PRMT1, the gene was cloned into the pGAPDH vector using enhanced green fluorescent protein (EGFP) as a marker [19]. The pGAPDHgPRMT1 plasmid was transfected into live cells of A. castellanii. Stable transfection was performed using the SuperFect transfection reagent (Qiagen) following the manufacturer’s recommendations. Transfected cells were transferred to 50 μg neomycin-containing G418 media and grown over several passages. The amoeba expressing EGFP was allowed to adhere to a cell culture dish, and the LSM 5 EXCITER Scalable confocal system (ZEISS, Hamburg, Germany) was used to observe the amoeba. EGFP- or DAPI (4′,6-diamidino-2-phenylindole) mediated fluorescence was performed using bandpass filters covering excitation and emission wavelengths of 500 to 530 nm and 360 to 460 nm, respectively.
Gene silencing
Small interfering RNA (siRNA) targeting PRMT1 of A. castellanii was synthesized by Sigma-Proligo (Boulder, Colorado, USA), based on its cDNA sequence (Table 1). The siRNA designed against AcPRMT1 (final concentration of 100 nM) was transfected into live A. castellanii trophozoites at a cell density of 4×105 per well using the SuperFect transfection reagent (Qiagen) following the manufacturer’s protocol.
RESULTS
Identification of AcPRMT1
To identify the epigenetic regulator in A. castellanii, we used the basic local alignment search tool (BLAST) and KOG (euKaryotic Orthologous Groups) analysis from our existing cDNA library, and identified a full-length open reading frame of AcPRMT1 (GenBank accession no. KT345168). AcPRMT1 has 352 amino acids with a predicted molecular mass of 38.7 kDa. Based on homology searches, the deduced amino acid sequences of the AcPRMT1 cDNA showed 42.6% similarity with AcPRMT5 (GenBank accession no. KT345169) (data not shown). Amino acid sequence alignment of AcPRMT1 with that of Schistosoma japonicum, E. histolytica, Dictyostelium discoideum, P. berghei, and Homo sapiens showed sequence similarity (Fig. 1). Putative AcPRMT1 encodes a SAM-dependent methyltransferase PRMT-type domain (boxed area).
Gene expression during encystation
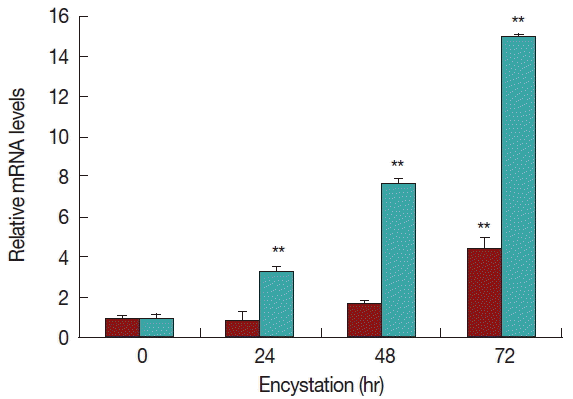
Real-time PCR analysis was used to detect the transcript levels of AcPRMT1 during encystation of A. castellanii. The expression levels of AcPRMT1 were highly increased after induction of encystation from 24–72 hr (Fig. 2). The previously identified PRMT5 of A. castellanii (AcPRMT5) also showed increased expression patterns during encystation [19]. However, AcPRMT1 was expressed higher than AcPRMT5 during encystation of A. castellanii (Fig. 2).
Intracellular localization of AcPRMT1
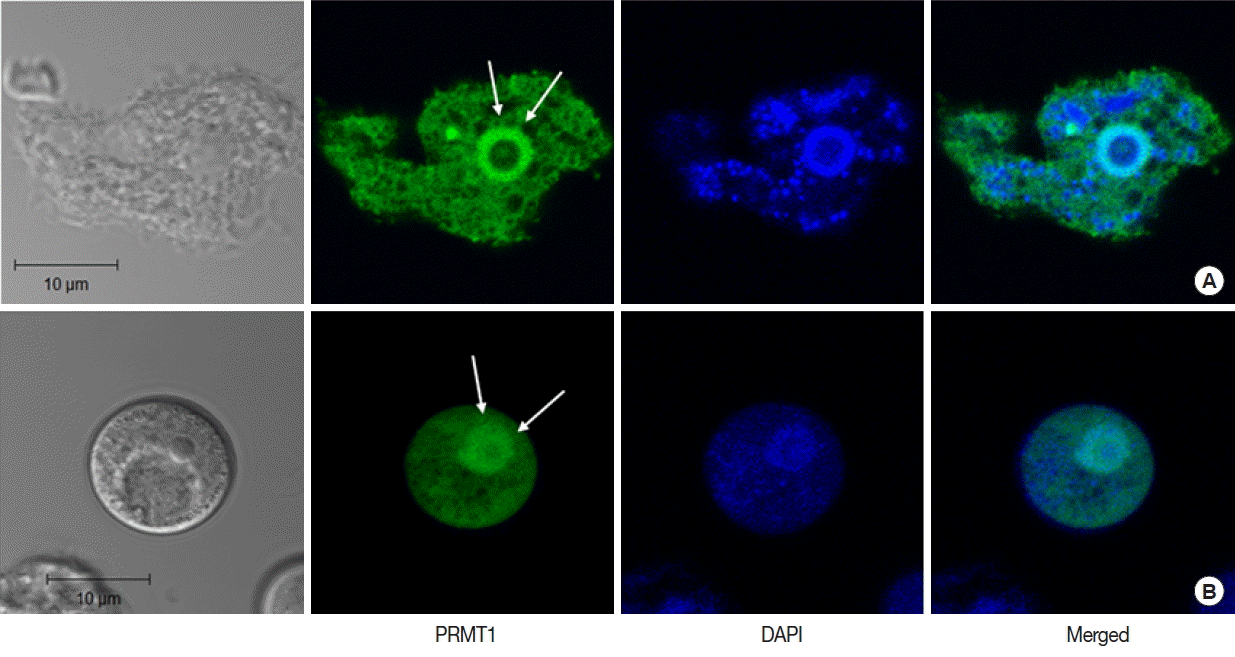
In A. castellanii transfected with EGFP-tagged AcPRMT1, strong GFP fluorescence was detected in the cytoplasm and the nucleus (Fig. 3). Previously it was found that the EGFP-AcPRMT5 fusion protein was mainly localized in the nucleus of Acanthamoeba [19]. However, AcPRMT1 was localized in both the cytoplasm and the nucleus of Acanthamoeba trophozoite (Fig. 3A) and cyst (Fig. 3B). The localization of AcPRMT1 in the nucleus was confirmed by DAPI staining (Fig. 3).
Effect of AcPRMT1 on encystation of Acanthamoeba
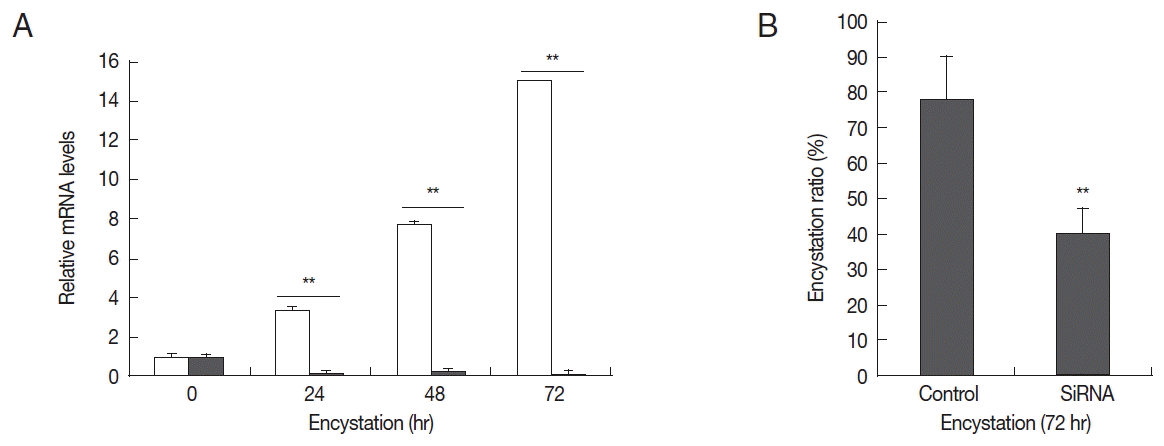
To determine the effects of AcPRMT1 on encystation of A. castellanii, post-transcriptional gene silencing by siRNAs was performed. After transfection of fluorescent tagged siRNA against AcPRMT1 (Table 1), the transfection efficiency of siRNA was determined to be 80% by fluorescent microscopy. Transfected trophozoites were transferred to the encystment media, and the effect of AcPRMT1 gene silencing on encystation of Acanthamoeba was investigated. As shown in Fig. 4A, the mRNA expression of AcPRMT1 was successfully decreased during encystation. Decreased expression of AcPRMT1 by siRNA affected the encystation ratio of Acanthamoeba. The number of mature cysts was significantly reduced in siRNA transfected cells (40.2%) compared to control cells (78.4%) (Fig. 4B).
DISCUSSION
Histone arginine methylation by PRMTs has been shown to play an important role in transcriptional regulation [20,21]. In this study, PRMT1 was identified for the first time in A. castellanii (AcPRMT1). We suggested that PRMT1 play an important role in encystation of Acanthamoeba based on the result that the cells transfected with siRNA against AcPRMT1 failed to form mature cysts (Fig. 4). AcPRMT1 was localized both in the cytoplasm and the nucleus (Fig. 3), while we have previously identified that AcPRMT5 was localized mainly in the nucleus [19]. However, the substrates of PRMT1 and PRMT5 of A. castellanii have yet to be identified. PRMT1 of P. falciparum was localized in both the cytoplasm and the nucleus, and this suggested the presence of potential substrates in both cellular compartments [13].
PfPRMT1 methylated histone H4 predominant at arginine 3 and formed monomethylarginine (MMA) and asymmetric dimethylarginine (ADMA) [13]. The authors also previously confirmed the presence of histone H3 arginine 17 methylation in P. falciparum, a potential substrate for the CARM-like PRMT. PRMT1 of S. mansoni was able to specifically methylate histone H4, but not histone H3 [22]. Unfortunately, the sequence of histone H4 of A. castellanii Castellani has not yet been reported. Also, histone H4 arginine 3 position in H. sapiens was different from that in A. castellanii Neff (GenBank accession no. ELR24196). However, histone H3 arginine residues (Arg2, Arg8, and Arg17) of A. castellanii Castellani and A. castellanii Neff (no. ELR19918) are the same with those of humans (no. AAN39284) (data not shown). It is highly supposed that AcPRMT1 is able to methylate histone H3 arginine 17 of Acanthamoeba (MARTKQTARKSTGGKAPRKMAS) similar to CARM-like PRMT.
PRMT1 mediated dimethylation of histone H4 arginine 3 is associated with gene activation [23], while the methylation of histone H4 arginine 3 by PRMT5 is associated with gene repression [24]. The authors hypothesized that AcPRMT1 and AcPRMT5 regulate the transcriptional levels of encystation associated genes by histone modification. Furthermore, we hypothesized that AcPRMT1 is associated with gene expression, while AcPRMT5 is associated with gene repression. AcPRMT1 was expressed higher than AcPRMT5 during encystation (Fig. 2), which suggested that there are various active genes associated with encystation.
On the basis of these results, further research is required to obtain target substrates and specific inhibitors of AcPRMT1. We predict that PRMT1 plays a key role in the expression of certain resistance genes in encysting Acanthamoeba. Further research will lead to the development of anti-amoebic drugs for human Acanthamoeba infections.