INTRODUCTION
Deer keds Lipoptena spp. (Hippoboscidae, Lipopteninae) are blood-sucking obligate ectoparasites of almost exclusively Cervidae (deer) and Bovidae (cattle, goats, chamois, antelopes, etc.), and can occasionally bite humans [1–3]. Indeed, the Lipoptena genus includes about 30 species spread worldwide [1]: most of them occur in the Palearctic region, mainly in continental Europe and Asia, while 8 species are native to far East Asian countries. Five species have been recorded in America, with 4 of them native to this continent [4]. Fragmentary information is available on the species accounted for African countries [5,6] (Supplementary Table S1).
In severe infestations, Lipoptena spp. may be responsible for anemia and skin lesions and may be involved in the transmission of several pathogens [7,8].
In Europe, 5 species of Lipoptena have been recorded: Lipoptena capreoli Róndani, 1878, Lipoptena couturieri Séguy, 1935, Lipoptena arianae Maa, 1969, Lipoptena cervi (Linnaeus, 1758), and Lipoptena fortisetosa Maa, 1965 [9]; however, the presence and the geographical range of the first 3 species need to be confirmed.
Lipoptena cervi may be considered the oldest deer ked in Europe as the relationship of this species with wild ungulates dates back to more than 5,000 years ago, being found in the remains of a Late Neolithic human mummy discovered in a glacier in the Alps [10]. It has a wide distribution in Europe and has currently been reported from more than 20 countries [11]. Lipoptena cervi has been recorded on Cervus elaphus, Dama dama, Alces alces, Rupicapra rupicapra, Capreolus capreolus, Moschus moschiferus [9] and it has the potential to transmit bacteria e.g., Bartonella spp., Borrelia spp. [12–14], Anaplasma spp., Ehrlichia spp., and Rickettsia spp., and protozoans, e.g., Babesia spp., Theileria spp., Hepatozoon spp. [15–17].
Lipoptena fortisetosa was originally recorded in Japan from the sika deer Cervus nippon [18–20], and is considered quite restricted to this ungulate, although it has been occasionally collected from a bird Emberiza spodocephata [21]. It has also been reported from the Siberian roe deer Capreolus pygargus in South Korea [22] and in Capreolus capreolus in Kazakhstan [23]. This species was first reported in Europe about 50 years ago when it was found in the Czech Republic [24] and later in the Moscow district in Russia [25]. Afterward, from the 80s’ to date, it has been confirmed and/or recorded in 12 countries, i.e., Czech Republic, Poland, Moldavia, Germany, Switzerland, Lithuania, Romania, Austria, Belarus, Slovak Republic, Moscow-district, and Estonia [26]. The range of L. fortisetosa has expanded in the southern part of Europe, including Italy, where it has very recently been reported [27,28]. In Europe, L. fortisetosa attacks mainly deer [29,30], and occasionally cattle [31], goats, sheep [29,30], dogs [32,33], and humans, as reported in Germany [34], Estonia [26], and Slovakia [35]. Lipoptena fortisetosa has been found to mechanically carry pathogens, i.e., Coxiella-like bacteria (CLB), Theileria luwenshuni, and Theileria ovis [36]. In addition, very recently, both L. cervi and L. fortisetosa specimens from Poland were found positive to Trypanosoma DNA [37].
In Europe, L. fortisetosa appears to share with L. cervi approximately the same ungulate species as the host group (above listed) and roughly the same territory [26]. However, it is unclear whether the native host (i.e., the sika deer Cervus nippon) of L. fortisetosa played a role in spreading the Asian species to Europe or the parasite propagated independently, as already speculated [38], or to which extent human activities might have helped the ked expansion. Lipoptena fortisetosa likely dispersed widely and settled in Europe quite quickly, so that possible changes in genetic constitution compared to the Asian indigenous populations may be hypothesized.
Molecular investigations coupled to morphological analysis, as well as the ecological requirements, help to provide more insight into the perspective of the integrated taxonomy concept [39].
In order to state whether there are sharp differences between Italian and Asian individuals, we developed a morpho-molecular approach. In particular, we investigated the phylogenetic interrelationship of the mitochondrial cytochrome c oxidase subunit 1 (CO1) gene haplotypes of Lipoptena individuals sampled in Italy-analyzing them in a wider context together with the DNA sequences of conspecifics available in the NCBI GenBank and morphologically analyzed the Italian population of L. fortisetosa. We also focused on the observation of some stable key-characters (terminalia) and compared them with the original description of the species, as well as with the indigenous L. cervi species, as additional documentation. Finally, the presence of the sika deer in Europe was retraced in order to discuss the possible route traveled by the ectoparasite from Asia and the eco-biological factors that may have enhanced its settlement.
MATERIAL AND METHODS
Ethics statement, specimens and processing
All animal handling procedures followed all regional, national, and institutional guidelines.
From a total of 312 Lipoptena specimens, previously collected from 3 species of wild ruminants [28], belonging to Lipoptena cervi and Lipoptena fortisetosa, the following were selected from different host species: 10 flies each from 5 Cervus elaphus hosts (total 50 specimens: 30 and 20 specimens belonging to L. cervi and L. fortisetosa, respectively); 10 flies from 3 Capreolus capreolus hosts (total 30 specimens, all belonging to L. fortisetosa), 10 flies from 1 Dama dama host (total 10 specimens, all belonging to L. fortisetosa), and frozen at −20°C, until DNA extraction. Genomic DNA was extracted individually from the abdomens using the Nucleospin Tissue kit (Macherey-Nagel, Amsterdam, Netherlands) in accordance with the manufacturer’s instructions. The extracted DNA was eluted in 50 μl of distilled water and the samples were stored at −20°C, pending molecular analysis. PCR amplifications were performed in a CFX96 thermal cycler (Bio-Rad, Hercules, California, USA.) using 10 μl of Phire Reaction Buffer 5X (Thermo Scientific, Waltham, Massachusetts, USA), 0.4 μl of dNTPs (200 μm) (Qiagen, Germantown, Maryland, USA), 1 μl of specific primer pairs (10 μm), 0.4 μl of Phire Hot Start II DNA Polymerase 1 U (Thermo Scientific), and 5 μl (approximately 100 μg) of genomic DNA per reaction. A blank control (pure water instead of genomic DNA) was included in each PCR run.
An approximately 710-bp gene fragment of CO1 was amplified using primers LCO-1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO-2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) [40]. The cycling parameters were: 2 min denaturing at 94°C, followed by 35 cycles of 30 sec at 94°C, 30 sec at 56°C and 60 sec at 70°C, and final extension of 7 min at 70°C. PCR products were run on 1.2% agarose gel, and positive samples purified with exonuclease I (EXO I) and thermosensitive alkaline phosphatase (FAST AP) (Fermentas, Waltham, Massachusetts, USA) enzymes, in accordance with the manufacturer’s instructions.
PCR products were directly sequenced in both directions using the ABI PRIMS BygDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California, USA) with the same primers as the respective PCR reactions, in accordance with the manufacturer’s instructions. The sequences obtained were determined using an ABI PRISM 3130 Genetic Analyser (Applied Biosystems), chromatograms were inspected by eye using FinchTV (https://digitalworldbiology.com/FinchTV) and primer regions plus bad-quality regions were removed. Once the sequences were cleaned up, each sequence was compared with the Lipoptena spp. homologous nucleotide sequences available in the GenBank database using the BLAST program (Basic Local Alignment Search Tool; https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_L+OC=blasthome). The 19 sequences having the highest percent similarity with our sequences and labelled as Lipoptena CO1 in Genbank were then sampled and gathered in a FASTA file with our own sequences. The new sequence dataset was aligned using the CLUSTALW implementation of BIOEDIT, version 7.0.5 (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) and the alignment adjusted manually, if necessary. Once the sequences were aligned, the absence of stop codons was checked. Phylogenetic analysis of the obtained sequences and homologous sequences from GenBank were performed using the maximum likelihood method in MEGA, version 7.0.9 (https://www.megasoftware.net). Bootstrap confidence values for the branching reliability were calculated with 10,000 replicates.
All specimens of L. fortisetosa and L. cervi intended for molecular analysis were morphologically identified, based on Maa’s original description [19] and a recent taxonomic key [9]. Other specimens were processed for Scanning Electron Microscope (SEM) observations, according to the procedures previously described [28] to further examine male and female terminalia. Description of terminalia features follows the terminology and nomenclature reported by Maa and Peterson [41].
RESULTS
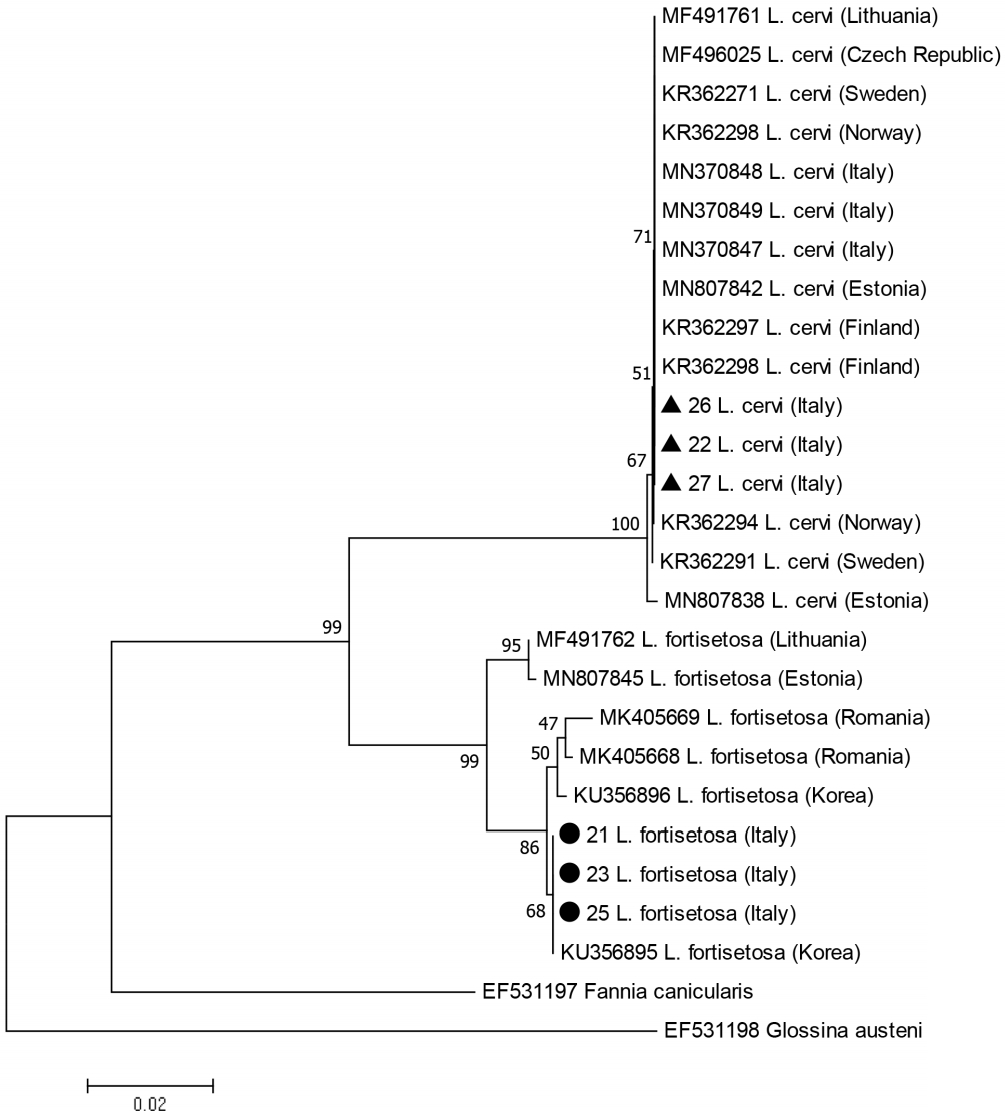
A total 60/90 (66.7%) of the specimens provided good quality PCR fragments and sequences for the CO1 gene. After alignment with the homologous sequences of Lipoptena spp. available in GenBank, 2 sets of sequences were identified, one with the mean percentage of identity of 89% with L. cervi and 92% with L. fortisetosa, and one with 97% with L. cervi and 86% with L. fortisetosa. Two haplotypes were recorded from the 60 specimens sequenced in the present study and the phylogenetic analysis confirmed that these 2 haplotypes belonged to L. cervi and L. fortisetosa, respectively. The data matrix comprised 14 haplotypes of L. cervi (one haplotype from the present study, 13 downloaded from GenBank) and 7 haplotypes of L. fortisetosa (one haplotype from the present study, 6 downloaded). The genetic distances ranged from 0.081–0.084 for L. cervi group sequences and 0.003–0.018 for L. fortisetosa group sequences.
While the clade L. fortisetosa appears to be monophyletic, the internal 2-subclade structure of this clade is poorly supported (low bootstrap values) as expected within the species. We will thus note essentially the following points: the single haplotype recorded from the Italian L. fortisetosa specimens was closely related to 2 Central European haplotypes and 100% identical to one of the 2 haplotypes (KU356895) found in Korea (Fig. 1).
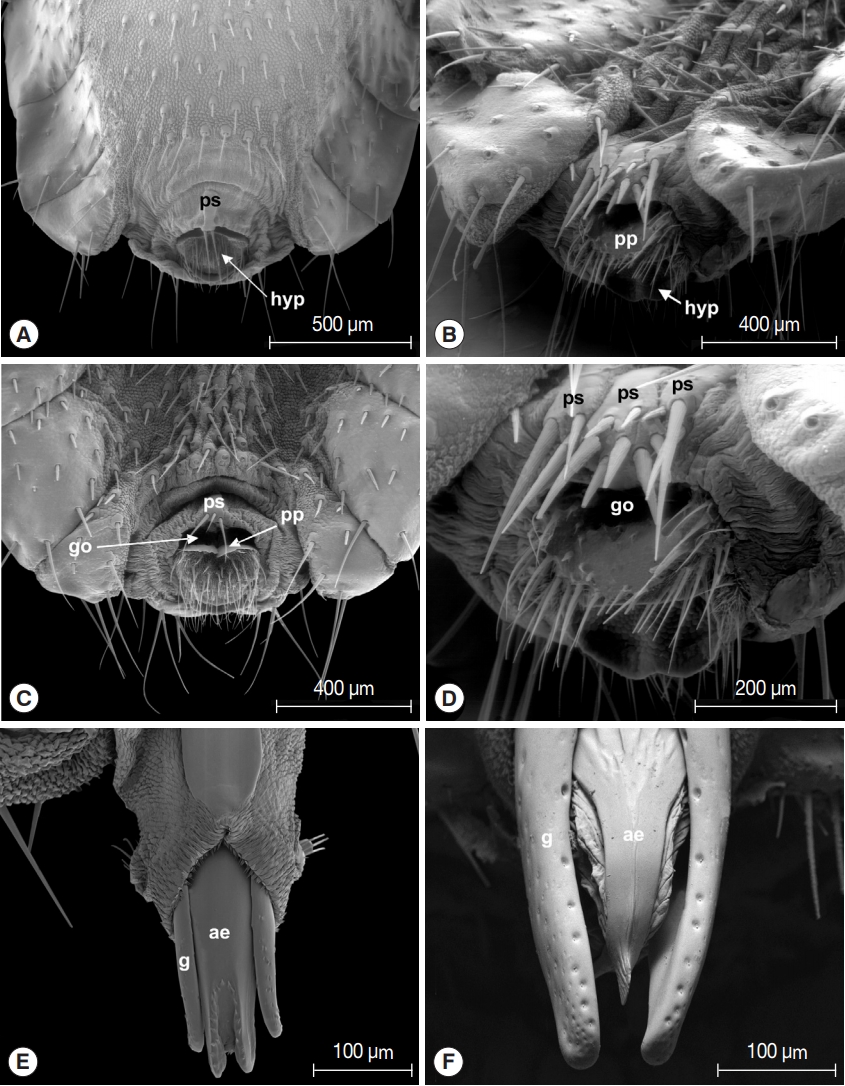
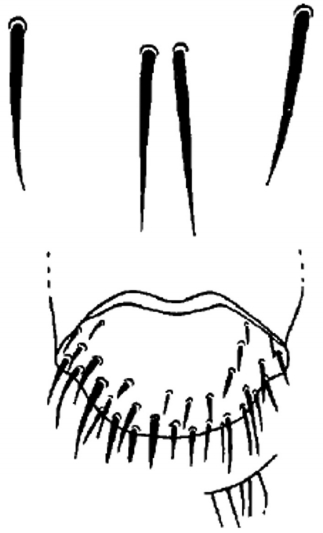
Morphological investigations showed that female terminalia of L. fortisetosa are characterized by a typical pregenital sclerite that is peg-like and bears 2 or 3 strong bristles (Fig. 2A, C). The pregenital plate is elongated and lozenge-shaped, and the underlying hypoproct is covered by several bristles interspersed with an area densely hairy. The genital opening is clearly visible between the pregenital sclerite and the pregenital plate (Fig. 2C). Female terminalia of L. cervi showed the presence of 3 pregenital sclerites bearing several differently sized bristles (Fig. 2B, D). The central sclerite is bigger and with more numerous setae than the external 2; the pregenital plate shows many series of long setae arranged in the distal portion, while the hypoproct is completely bare. Male terminalia of L. fortisetosa (Fig. 2E) consist of 2 well-sclerotized and slender gonopods that guide the aedeagus. This latter is wider in the proximal part and ends with a bilobate tip provided with spines. In L. cervi, the gonopods (Fig. 2F) are similar to those of L. fortisetosa. However, the aedeagus is membranous in the proximal and lateral parts, while in the middle it is formed by 2 fused and sclerotized strips ending in a ridge tip. Morphological observations of L. cervi and L. fortisetosa strengthened the strong diversity between these 2 species [28] but, more importantly, demonstrate that the features of L. fortisetosa female terminalia are consistent with the original description by Maa [18] (Fig. 3).
Taking into account that terminalia are considered stable features that allow a correct morphological identification at species-specific level in Diptera [42], including hippoboscids [19,43], this supports the above confirmation that the boundaries between the 2 species (L. fortisetosa and L. cervi) are correct based on morphology and molecular data. Therefore, we can conclude that the individuals of which we only have the sequences (GenBank) belong to the same species.
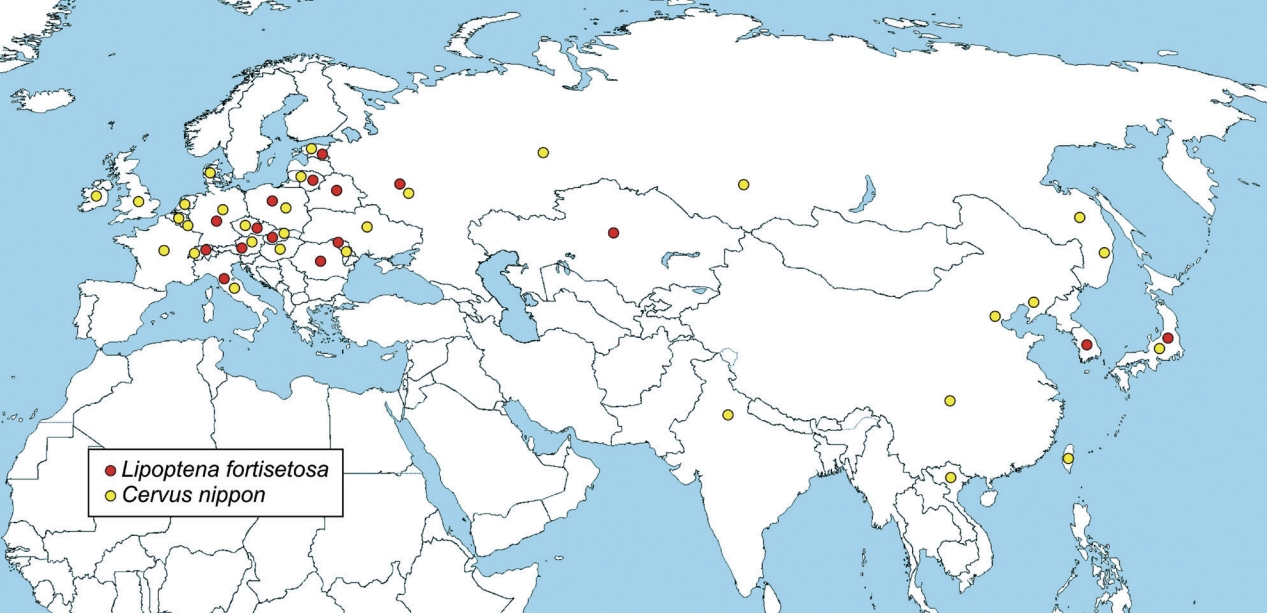
Lipoptena fortisetosa has been introduced in Europe probably with Cervus nippon, the sika deer [28,33,44], during the last 150 years of restocking of deer in the Continent, apparently, since 1893, and probably a number of times [44]. Sika deer has successfully settled in the European fauna thanks to its high potential to compete with autochthonous species and readiness to hybridize with native red deer, as demonstrated by the presence of hybrids of sika with red deer in several countries [45–49]. Sika deer (or hybrids) is currently present in 20 European countries, including Italy, where it was recently reported [50]. Spreading through Europe, sika deer has likely carried and disseminated its ectoparasites, including L. fortisetosa that is currently recorded in 13 European countries (Fig. 4), Italy included [27].
DISCUSSION
According to the present results, while L. cervi confirms previous findings [9], we cannot exclude a scenario depicting a recent colonization(s) of L. fortisetosa in Europe. According to the latest studies, the hypothesis of geographically distinct CO1 lineages should be rejected at the Central Europe level [26]. Our study suggests that this hypothesis should also be rejected at a global level. However, we were unable to determine the time of colonization or to identify the actual host that introduced L. fortisetosa from Asia to Europe. In fact, this parasite has been collected from many different cervids, e.g., red deer (Cervus elaphus) [27,28], Manchurian elk (Cervus elaphus xanthopygus), Maral red deer (Cervus elaphus maral), fallow deer (Dama dama) [34], Korean water deer (Hydropotes inermis) [36], roe deer (Capreolus capreolus) [23], Siberian roe deer (Capreolus pygargus) [22], both in Europe and in Asia. The possibility of recent colonization by L. fortisetosa specimens from cervids purchased for the restocking of deer farms, or in captive hosts in the Oriental region transferred to fenced areas for recreational or conservation purposes, cannot be excluded. We also suggest, as a minor hypothesis, the relatively recent migration to Italy of sika or hybrid individuals from neighboring countries that may have transmitted the ectoparasite joining red deer groups.
This study doesn’t reject the hypothesis of the recent colonization of Italy by L. fortisetosa from Asia as no obvious and stable morphological and molecular differences were observed in the populations from the 2 regions. However, such a hypothesis requires further study, including a straightforward analysis of numerous specimens from Asian and European countries. In line with other authors’ suggestions [26], population genetic analyses of Asian and European keds’ populations are required to evaluate whether the colonization scenario is likely or not. Moreover, wider investigations aimed at comparing genetic make-up of populations from different countries, host and parasite distribution, together with morphological differentiation, might resolve phylogenetic relationships of this neglected group within a desirable integrated taxonomic framework as applied in other groups of insects [51,52].
Due to the ability of L. fortisetosa to parasitize a wide range of homeothermic animals, there are no obvious limitations to its further expansion. Given such possibility, the potential for transmitting pathogens, and the frequent attacks reported among humans [26], more in-depth investigations are required on Lipoptena species with a One Health perspective.