INTRODUCTION
Alveolar echinococcosis (AE) is a zoonotic disease that primarily affects the liver but has the potential for local invasion and metastasis to brain, lung and bones. AE is distributed worldwide, mainly in the northern hemisphere [1,2]. Even though AE is a benign disease caused by parasites in histopathology, it shows the growth characteristics of malignant tumors [2]. Almost all AE originates in the liver and can spread to distant organs such as the lungs, brain, and kidneys through blood circulation [3,4]. Human are not suitable intermediate hosts for E. multilocularis, and the presence of the protoscolices is rarely observed in the lesions. In the present case, we report a case of primary hepatic AE with lung and brain metastases. The patient had a history of Mycobacterium tuberculosis infection, and the imaging features of lung lesions showed pulmonary abscess. As a result, we were misdiagnosed. The patient underwent radical resection of liver and lung lesion and was treated with albendazole regularly after operation. The patient had multiple AE metastases in the brain about one year after the lung lesion surgery. Eventually, the patient lost a chance to treat due to multiple intracranial AE lesions.
CASE RECORD
Written informed consent was obtained from patient and the article was approved by the Ethics Committee of the Affiliated Hospital of Qinghai University (AF-RHEC-0018-01).
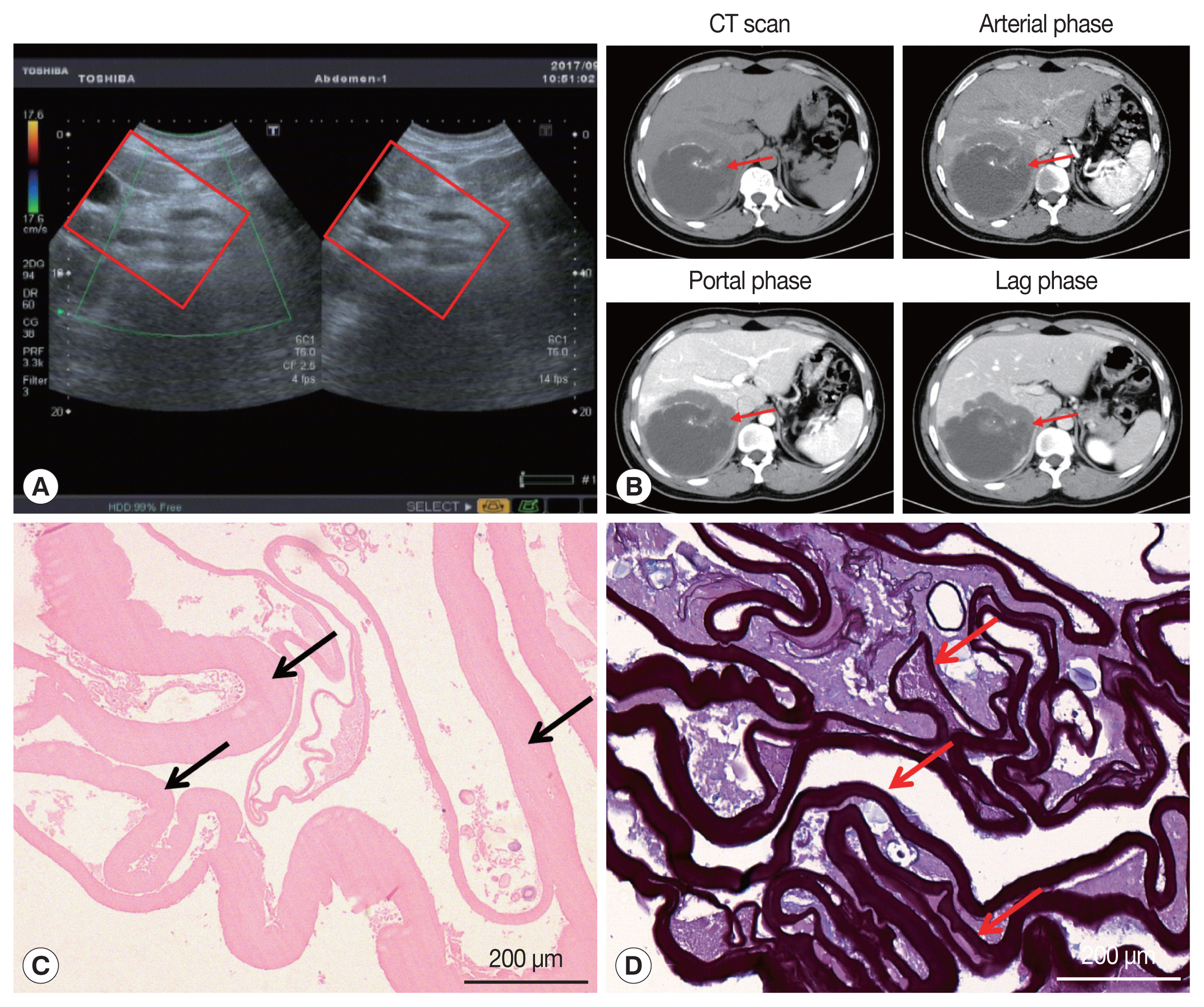
On January 3, 2017, a 34-year-old Tibetan Chinese man came to the Department of Neurosurgery at the Qinghai University Affiliated Hospital for treatment with severe upper abdominal pain. The patient lived in pastoral areas of Qinghai Province for a long time and had been in good health before. Physical examination touched a mass in the upper abdomen, which was 18.0×16.0 cm in size, accompanied by tenderness. Ultrasound Color Doppler of the upper abdomen presented a 11.6×11.2 cm mixed mass in the right posterior lobe of the liver (Fig. 1A). The dynamic 3-phase Computed Tomography (CT) examination of the liver demonstrated a round lesions (about 9.6×8.9 cm) in the liver S7-6 segment, with a thick wall, and a CT value of about 16 HU. Calcification could be observed in the lesion, and no enhancement was seen in the lesion after enhancement (Fig. 1B). Chest X–ray and laboratory findings were all within normal limit. Additionally, hydatid serological IgG ELISA remained negative. Stool examination showed no parasites or eggs. Based on these findings, the patient was diagnosed as hepatic AE. The patient underwent right hepatectomy and cholecystectomy on September 6, 2017. During the operation, there existed obvious adhesion between the lesion and the lateral abdominal wall, which invaded the diaphragm and lateral peritoneum. Postoperative pathological examination confirmed AE disease. The histopathological examination showed obvious laminated layer (Fig. 1C), intensely colored by the periodic acid-Schiff stain without a germinal layer (Fig. 1D). These morphological findings verified AE. The patient fully recovered after surgery. The patient was treated with albendazole (400 mg twice a day) orally for 2 year.
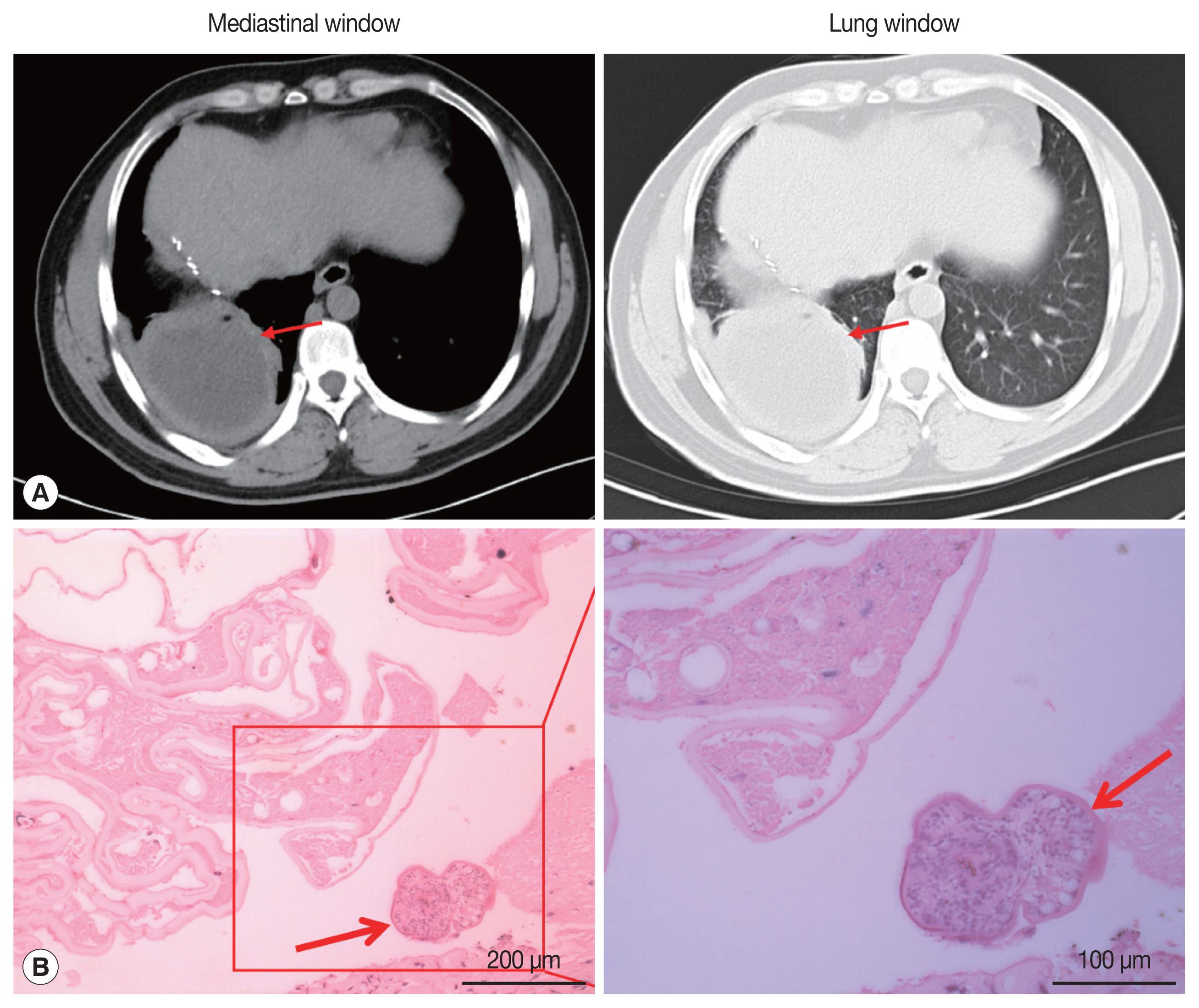
On May 13, 2019, the patient came to our hospital due to cough and chest pain for 15 days. The patient had hemoptysis once with obvious weight loss. Tissue components were found in the patient’s sputum. Oxygen saturation was about 60%. Chest CT showed an encapsulated lesion (about 8.4×6.9 cm) in the lower lobe of the right lung, considering the possibility of abscess formation (Fig. 2A). CT scan of brain showed no abnormalities. The patient’s hydatid serological IgG test and γ - interferon release test remained positive. In addition, any binding bacteria were not in sputum. Laboratory examination proved that the C-reactive protein was 20.50 mg/L (normal value 0–6), and the erythrocyte sedimentation rate was 30 mm/hr (normal value 0–15). The patient was considered to have a lung abscess caused by previous tuberculosis infection. The patient underwent radical resection of the lower lobe of the right lung on May 21, 2019. During the operation, there existed obvious adhesion in the right thoracic cavity, and an 8.0×8.0 cm abscess cavity was seen in the basal segment of the right lower lobe, which was filled with purulent fluid. The lower lobe and the diaphragm were adhered with no obvious boundary. The right lower pulmonary ligament, pulmonary artery, pulmonary vein and bronchus were ligated and cut off. The diaphragmatic adhesions were carefully separated and the lesions were completely removed. The pathological sections revealed the laminated membranes and protoscoleces (Fig. 2B). The patient’s pulmonary complications disappeared and pulmonary function completely recovered after surgery. we recommended that the patient to take albendazole orally (400 mg twice a day) for at least 2 years.
On March 30, 2020, the patient came to the neurosurgery department of our hospital again due to unprovoked dizziness and headache for 2 weeks. The patient suspected that he had an upper respiratory tract infection and took oral medication to treat it by himself (the specific medication was unknown). In the past 1 week, accompanied by nausea and vomiting, the patient had frequent headaches and blurred vision in his right eye. Physical examination of right limb muscle strength gave grade IV, and unclear vision in right eye. Brain CT images showed multiple round lesions in the brain parenchyma (Supplementary Fig. S1). According to MRI examination, there were multiple short T2 and other T1 signal lesions in the brain parenchyma with ring enhancement in the brain parenchyma, and obvious edema around the lesions (Fig. 3). The patient’s vital signs and laboratory tests were in the normal range. Combined with the patient’s medical history, the patient was clearly diagnosed as cerebral AE metastasized from the primary lesion. The multiple AE lesions in the brain were in the regions difficult to remove by operation. In addition, the patient had a grand mal seizure during hospitalization. When informing the current condition to his family members, they gave up further treatment and discharged the patient. At discharge, we recommended that the patient to take albendazole 400 mg twice a day for life
DISCUSSION
Alveolar echinococcosis is a serious and fatal helminth disease with an increasing incidence in endemic areas [5]. E. multilocularis adults mainly parasitize in the intestines of foxes, wolves and dogs, and the eggs excreted can contaminate food and water and infect human beings. The incubation period of AE infection is about 5–15 years, and there is no early clinical symptoms. The disease is usually diagnosed in the late stage, and only a small number of patients can undergo the complete surgical resection [6]. The diagnosis of AE needs to be combined with epidemiological history, clinical manifestations, imaging examination and serological examination [7].
According to its life cycle, human AE is almost 100% primary in liver, and can be transferred to lung, brain, heart, peritoneum and other parts through blood and lymphatic system in later stage [8–11]. Central nervous system involvement is considered to be a marker of the terminal stage of AE [12]. Additionally, our patient also shows obvious lung and brain metastases. Ultrasound Color Doppler has a high sensitivity in the diagnosis of hepatic vesicular hydatid disease and is often used as the first choice for preliminary diagnosis [13,14]. CT and MRI have important clinical value in diagnosis, postoperative follow-up, and judgment of calcification of AE [15,16]. The lesions of AE show no obvious boundary on ultrasound, CT, and MRI [17]. Our patient showed evident calcification on CT in the liver, with unclear peripheral boundaries and uninvaded portal veins. During the operation, it could be observed that the portal vein was not invaded, while the diaphragm was visibly violated. The patient underwent radical resection and long-term oral albendazole treatment. Despite these treatments, the patient was diagnosed with lung AE 2 years later.
Hepatic AE metastasis to the lung is relatively high, observed in 7–20% of the cases [18]. The clinical symptoms of lung AE are mostly hemoptysis, chest pain, cough with sputum, and exertional dyspnea. Our patient also has these symptoms. However, CT imaging of the patient’s lung lesions showed signs of lung abscess, with a thicker cyst wall, similar to the imaging findings of cystic echinococcosis [19]. The patient’s serum hydatid IgG and γ-interferon release test were positive. Imaging examination found no new lesions in the patient’s brain and liver. As a result, the patient’s AE was misdiagnosed as a lung abscess caused by old tuberculosis until it was confirmed by pathological examination. During the operation, the lung lesions were observed to adhere to the surrounding lung tissues, and there were tangible substances in the patient’s sputum, which may be caused by the lesions invading the bronchus. Although serological test has low sensitivity and specificity in the diagnosis of hydatid disease, it is useful in the diagnosis of hydatid disease and postoperative follow-up [20].
The clinical symptoms of brain AE depends on the degree of damage to central nervous system, including common symptoms of increased intracranial pressure, epilepsy, neurological disorders (such as blurred vision and paralysis), and cranial nerve palsy [21]. The CT and MRI of cerebral AE are mostly solid or multilocular cystic masses with clear boundaries, accompanied by calcification and peripheral edema [4]. Our patient also presented obvious nervous system involvement. CT and MRI of the patient showed multiple solid intracranial lesions with edema. The incubation period of AE infection is about 5–15 years, during which there are no obvious clinical symptoms. The patient’s brain lesions may have been metastasized due to liver lesion or rupture of a lung abscess. The patient currently depend on taking albendazole oral treatment for a long time. Albendazole can penetrate the blood-brain barrier more than mebendazole and is also the first choice of chemotherapy drugs for brain AE [22].
AE is a potentially fatal parasitic disease which is often misdiagnosed. The diagnosis depends on the comprehensive judgment by several diagnostic methods including imaging, serology, epidemiology, and histopathology. For metastatic AE, especially when the imaging features of the metastatic lesions are not typical, the previous history of echinococcosis provides great reference value for its diagnosis. Unfortunately, our patient has multiple brain AE metastases after 3 years of diagnosis. Currently, we only hope that relying on chemical drug treatment can enhance the quality of life of the patient.
Supplementary Information
Supplementary Fig. S1.
Head CT examination. Multiple space-occupying lesions with edema in brain parenchyma (red arrows)