AbstractThe Chinese edible frogs, Hoplobatrachus rugulosus (n=20), and the striped snakehead fish, Channa striata (n=34), were purchased from local markets in 3 administrative regions of Cambodia (Phnom Penh, Pursat, and Takeo Provinces) from May 2017 to April 2019, and their infection status with Gnathostoma sp. larvae was investigated. The frogs and fish were transported to the laboratory with ice and examined using the artificial digestion method. Advanced 3rd-stage larvae (AdL3) of Gnathostoma spinigerum, 24 in total number (1–6 larvae/frog), were detected from 6 (60.0%) out of 10 frogs purchased from Phnom Penh. No gnathostome larvae were detected in 10 frogs purchased from Takeo Province and 34 snakeheads from Phnom Penh, Pursat, and Takeo Provinces. AdL3 isolated from the frogs were 2.55–3.90 mm long and 0.31–0.36 mm wide. They had a characteristic head bulb (0.081×0.191 mm in average size) with 4 rows of hooklets, a muscular long esophagus (0.950–1.230 mm long), and 2 pairs of cervical sacs (0.530–0.890 mm long). The average number of hooklets in the 1st, 2nd, 3rd, and 4th rows was 41, 45, 48, and 51, respectively. These features were consistent with G. spinigerum AdL3. By the present study, it has been first confirmed that the Chinese edible frog, H. rugulosus, from Phnom Penh serves as a second intermediate host for G. spinigerum, although their intensity of infection was not so high compared to other previously reported localities.
Parasites in the genus Gnathostoma (Nematoda: Gnathostomidae) are clinically important as etiologic agents of foodborne parasitic zoonoses in humans. About 13 species are currently recognized to be valid. Among them, 6 species, i.e., G. spinigerum, G. nipponicum, G. doloresi, G. hispidum, G. malaysiae, and G. binucleatum, are known to be able to infect humans [1]. Human gnathostomiasis is caused by infection with larval gnathostomes, which is characterized clinically by creeping eruption in subcutaneous and intermuscular tissues due to migrating larvae [1]. It is also known that Gnathostoma larvae occasionally invade the visceral organs, including the liver, lungs, eyes, and even the brain [1,2].
Human gnathostomiasis is known to be prevalent in Southeast Asia, including Thailand, Lao PDR, and Myanmar, but this nematode infection is very rare in Cambodia [3–11]. Only 1 case of ocular gnathostomiasis was reported in Cambodia [12]. Larval gnathostomes were recovered from Asian swamp eels, Monopterus albus, in Cambodia, which were molecularly identified as G. spinigerum [13]. Chai et al. [14] also reported the infection status of Asian swamp eels from Cambodia with larval gnathostomes. In the present study, we examined the Chinese edible frogs, Hoplobatrachus rugulosus, and the striped snakeheads, Channa striata, purchased from local markets of Cambodia to determine their infection status with Gnathostoma larvae.
A total of 20 frogs and 34 snakeheads were purchased from local markets in 3 administrative regions, Phnom Penh Municipality (=Phnom Penh), Pursat, and Takeo Provinces of Cambodia from May 2017 to April 2019. All collected frogs and fish were transported with ice to the laboratory of the Department of Parasitology and Tropical Medicine, Gyeongsang National University College of Medicine, Jinju, Republic of Korea, and the length and weight of the frogs and fish were measured. Individual frog and fish was finely ground with a mortar with pestle. Then, the ground fish and frog meat was mixed with artificial gastric juice, and the mixture was incubated at 36°C for 2 hr. The digested material was filtered through a sieve (5×5 mm mesh), and washed with 0.85% saline until the supernatant became clear. The sediment was carefully examined under a stereomicroscope, and gnathostome larvae were collected based on their general morphological features. Some collected larvae were fixed with 10% hot formalin, cleared in alcohol-glycerin, and mounted in glycerin-jelly to observe the morphological characteristics.
A total of 24 larval gnathostomes (1–6 AdL3 larvae/frog) were detected in 6 (60.0%) out of 10 frogs from Phnom Penh in April 2019. However, no larval gnathostomes were detected in 10 frogs from Takeo Province and 34 snakeheads from Phnom Penh, Pursat, and Takeo Provinces from May 2017 to April 2019. AdL3 collected from the frogs were 2.55–3.90 mm long and 0.31–0.36 mm wide. They had a characteristic head bulb (0.081×0.191 mm in average size) with 4 rows of hooklets, a muscular long esophagus (0.950–1.230 mm long), and 2 pairs of cervical sacs (0.530–0.890 mm long) (Table 1). The average number of hooklets in the 1st, 2nd, 3rd, and 4th rows was 41, 45, 48, and 51, respectively (Fig. 1) (Table 1). These morphological features were consistent with the previous descriptions of G. spinigerum [1].
It has been first confirmed in this study that the Chinese edible frogs, H. rugulosus, from Phnom Penh, Cambodia serve as a second intermediate host for G. spinigerum. However, from Takeo Province, no gnathostome larvae were detected in 10 frogs. In Yangon, Myanmar, Chai et al. [15] detected AdL3 of G. spinigerum in 15 (75.0%) out of 20 Chinese edible frogs, with the infection intensity of 1–48 larvae (av. 10.5/frog). The prevalence (30.0%) of AdL3 of G. spinigerum in this study was very low compared with that (75.0%) of Chai et al. [15] in Myanmar. Moreover, the infection intensity (av. 4.0/frog) in this study was also lower than that (av. 10.5/frog) of Chai et al. [15]. Also in the case of Asian swamp eels, the prevalence and infection intensity of larval gnathostomes in Cambodia [14] was much lower than that in Myanmar [16]. Therefore, we could determine that the endemicity of larval ganthostomes is not so high in swamp eels and Chinese edible frogs from Cambodia compared with Myanmar.
In this study, we had a limitation that we could not investigate an enough number of frogs and snakeheads during the surveyed period. Only 20 frogs and 34 snakeheads could be examined due to a limitation in transportation of the samples with ice from Cambodia to the Republic of Korea. To reveal the exact infection status of larval gnathostomes in snakeheads and frogs in the future, extensive studies should be performed with a high number of samples collected from a variety of geographical regions in Cambodia.
With regard to the fish intermediate hosts of G. spinigerum, several species of fish have been reported [1]. However, in Cambodia, only 1 study has been available on larval gnathostome infections in Asian swamp eels [14]. In that study, 55.6% prevalence of larval gnathostomes was reported among the swamp eels purchased from Pursat Province, whereas 21 swamp eels purchased from Takeo Province and Phnom Penh gave all negative results [14].
In Myanmar, Chai et al. [17] detected larval gnathostomes in 58 (16.0%) out of 362 fish in 6 species, i.e., Channa lucius, Channa striata, Anabas testudineus, Chelon macrolepis, Heteropneustes kemratensis, and unidentified Channa sp., purchased from Yangon, Myanmar. In the forest snakeheads (C. lucius) and striped snakeheads (C. striata), the prevalence was 22.9% (24 of 105 fish examined) and 20.0% (11 out of 55 fish examined), and their mean intensities were 4.5 and 2.9 per infected fish, respectively. Jung et al. [18] reported 2 AdL3 of G. spinigerum in 1 out of 15 striped snakeheads purchased from a local market in a suburban area of Naypyidaw, Myanmar. However, in this study, we could not detect any larval gnathostomes in 34 striped snakeheads purchased from Phnom Penh, Pursat, and Takeo Provinces, Cambodia.
The results of this study suggest that 2 species of frogs and fish, i.e., the Chinese edible frogs and the Asian swamp eels, are potential sources of human infection with G. spinigerum in Cambodia. However, other possible intermediate or paratenic hosts of gnathostomes should be extensively surveyed in the near future. People having the food habit of consuming raw frogs or fish should pay attention to larval gnathostome infections in Cambodia.
ACKNOWLEDGMENTWe thank Jung-A Kim and Hee-Joo Kim, the Department of Parasitology and Tropical Medicine, Gyeongsang National University School of Medicine, Jinju, Republic of Korea, for their help in the examination of fish and frogs.
Conflict of interestThe authors have no conflicts of interest concerning the work reported in this paper.
REFERENCES1. Miyazaki I. Section III. Nematode Zoonoses. 33. Gnathostomiasis. An Illustrated Book of Helminthic Zoonoses. Tokyo, Japan. International Medical Foundation of Japan. 1991, pp 368-409.
2. Liu GH, Sun MM, Elsheikha HM, Fu YT, Sugiyama H, Ando K, Sohn WM, Zhu XQ, Yao C. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors 2020;13:616 https://doi.org/10.1186/s13071-020-04494-4
3. Bussaratid V, Dekumyoy P, Desakorn V, Jaroensuk N, Liebtawee B, Pakdee W, Wattanagoon Y. Predictive factors for Gnathostoma seropositivity in patients visiting the gnathostomiasis clinic at the hospital for tropical diseases, Thailand during 2000–2005. Southeast Asian J Trop Med Public Health 2010;41:1316-1321.
4. Jongthawin J, Intapan PM, Sanpool O, Sadaow L, Janwan P, Thanchomnang T, Sangchan A, Visaetsilpanonta S, Keawkong W, Maleewong W. Three human gnathostomiasis cases in Thailand with molecular identification of causative parasite species. Am J Trop Med Hyg 2015;93:615-618 https://doi.org/10.4269/ajtmh.15-0284
5. Hennies F, Jappe U, Kapaun A, Enk A. Gnathostomiasis: import from Laos. J Dtsch Dermatol Ges 2006;4:414-416 https://doi.org/10.1111/j.1610-0387.2006.05942.x
6. Vonghachack Y, Dekumyoy P, Yoonuan T, Sa-nguankiat S, Nuamtanong S, Thaenkham U, Phommasack B, Kobayashi J, Waikagul J. Sero-epidemiological survey of gnathostomiasis in Lao PDR. Parasitol Int 2010;59:599-605 https://doi.org/10.1016/j.parint.2010.08.007
7. Nomura Y, Nagakura K, Kagei N, Tsutsumi Y, Araki K, Sugawara M. Gnathostomiasis possibly caused by Gnathostoma malaysiae. Tokai J Exp Clin Med 2000;25:1-6.
8. Chai JY, Han ET, Shin EH, Park JH, Chu JP, Hirota M, Nakamura F, Nawa Y. An outbreak of gnathostomiasis among Korean emigrants in Myanmar. Am J Trop Med Hyg 2003;69:67-73 https://doi.org/10.4269/ajtmh.2003.69.67
9. Develoux M, Dekumyoy P, Baygon E, Aractingi S. Imported gnathostomiasis acquired in Myanmar. Med Mal Infect 2006;36:340-342 (in French). https://doi.org/10.1016/j.medmal.2006.01.011
10. Wai AP, Maw WW, Moe AC, Boonmars T, Nawa Y. Human gnathostomiasis in Myanmar: A review of local literature. Southeast Asian J Trop Med Public Health 2018;49:543-548.
11. Duong MC, Le PV, Pham ON, Huynh HQ. Cutaneous gnathostomiasis in Vietnam. Med J Aust 2020;212:252-253e1 https://doi.org/10.5694/mja2.50514
12. Hem S, Tarantola A, Chheang R, Nop P, Kerléguer A. First reported case of intraocular Gnathostoma spinigerum in Cambodia. Bull Soc Pathol Exot 2015;108:312-315 https://doi.org/10.1007/s13149-015-0453-2
13. Boonroumkaew P, Sanpool O, Rodpai R, Sadaow L, Somboonpatarakun C, Laymanivong S, Aung WPP, Un M, Laummaunwai P, Intapan PM, Maleewong W. Molecular identification and genetic diversity of Gnathostoma spinigerum larvae in freshwater fishes in southern Lao PDR, Cambodia, and Myanmar. Parasitol Res 2019;118:1465-1472 https://doi.org/10.1007/s00436-019-06292-z
14. Chai JY, Jung BK, Lee KH, Hong SJ, Khieu V, Na BK, Sohn WM. Infection status of Gnathostoma spinigerum larvae in Asian swamp eels, Monopterus albus, purchased from local markets in Cambodia. Korean J Parasitol 2020;58:695-699 https://doi.org/10.3347/kjp.2020.58.6.695
15. Chai JY, Jung BK, Ryu JY, Kim HS, Hong SJ, Htoon TT, Tin HH, Na BK, Sohn WM. Larval gnathostomes and spargana in Chinese edible frogs, Hoplobatrachus rugulosus, from Myanmar: Potential risk of human infection. Korean J Parasitol 2020;58:467-473 https://doi.org/10.3347/kjp.2020.58.4.467
16. Chai JY, Sohn WM, Na BK, Park JB, Jeoung HG, Hoang EH, Htoon TT, Tin HH. Larval Gnathostoma spinigerum detected in Asian swamp eels, Monopterus albus, purchased from a local market in Yangon, Myanmar. Korean J Parasitol 2015;53:619-625 https://doi.org/10.3347/kjp.2015.53.5.619
17. Chai JY, Jung BK, Lee KH, Ryu JY, Kim HS, Hong SJ, Htoon TT, Tin HH, Na BK, Sohn WM. Larval gnathostomes and zoonotic trematode metacercariae in fish from a local market in Yangon City, Myanmar. Korean J Parasitol 2020;58:701-707 https://doi.org/10.3347/kjp.2020.58.6.701
18. Jung BK, Lee JJ, Pyo KH, Kim HJ, Jeong HG, Yoon CH, Lee SH, Shin EH, Chai JY. Detection of Gnathostoma spinigerum third-stage larvae in snakeheads purchased from a central part of Myanmar. Korean J Parasitol 2008;46:285-288 https://doi.org/10.3347/kjp.2008.46.4.285
Fig. 1An advanced third-stage larva of Gnathostoma spinigerum detected in a Chinese edible frog, Hoplobatrachus rugulosus, purchased from a local market in Phnom Penh, Cambodia. The larva has a characteristic head bulb, muscular esophagus (E), intestine (I), and 4 cervical sacs (CC). Scale bar=0.5 mm. 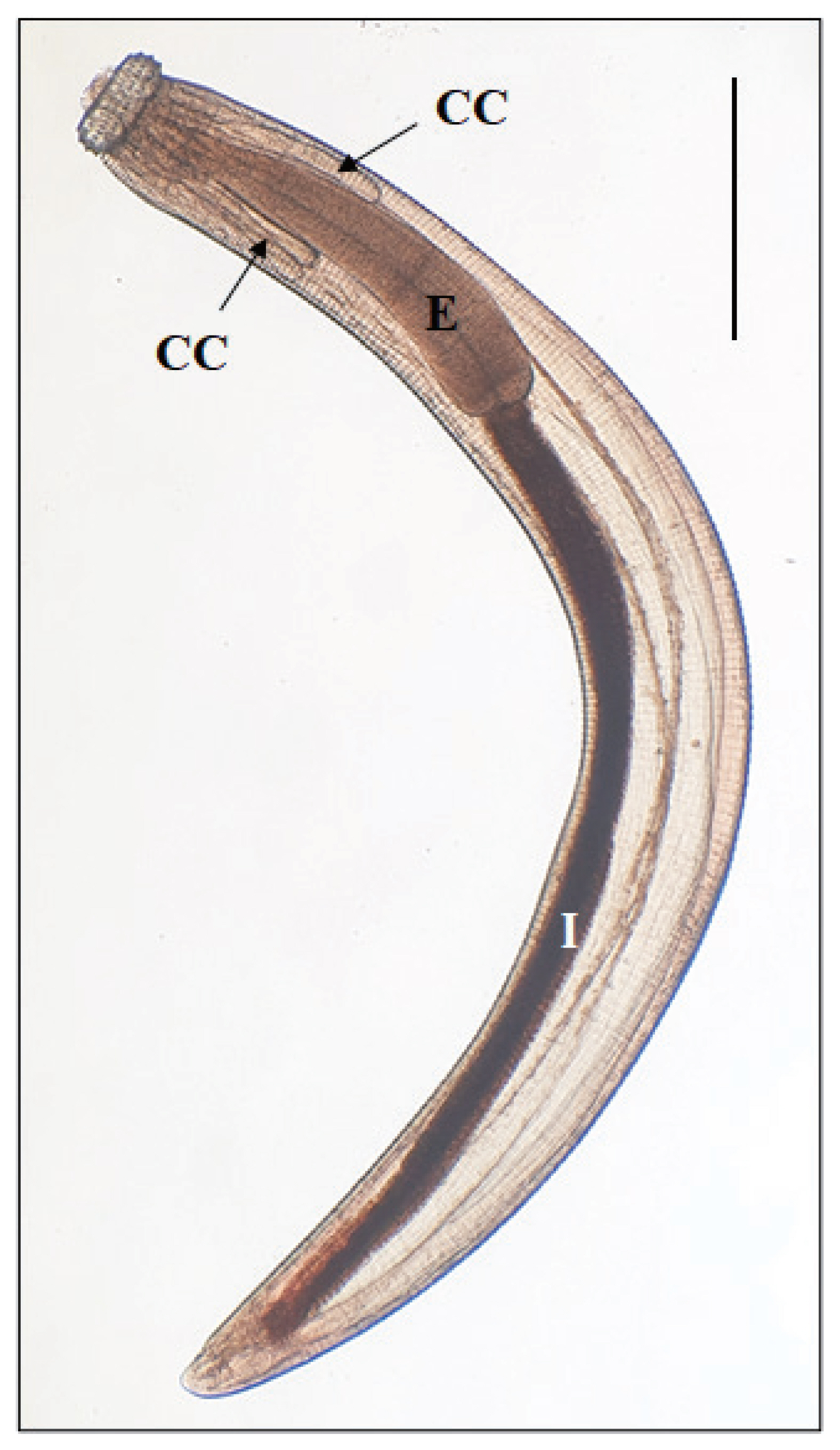
Table 1Measurements of the advanced 3rd-stage larvae of Gnathostoma spinigerum in Chinese edible frogs, Hoplobatrachus rugulosus, from Phnom Penh, Cambodia (present study), in comparison with a previous study in Myanmar
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||