INTRODUCTION
G6PD deficiency is the most common X-linked recessive hereditary enzymopathy in humans, which affects over 500 million people globally [1]. G6PD plays a crucial role in the first step of the pentose phosphate pathway (PPP), which is the only source of generating the co-enzyme, nicotinamide adenine dinucleotide phosphate (NADPH), in RBCs. The NADPH passes the electron to oxidized glutathione (GSSG) in the antioxidant pathway, producing the reduced glutathione (GSH). The GSH generation helps to protect cells from oxidative stress by removing the reactive oxygen species (ROS) [2]. G6PD activity is therefore crucial for protecting cells against oxidative damage. The gene encoding G6PD is highly polymorphic. Its mutations frequently cause clinical manifestation because of decreased enzyme activity and stability [3]. Most G6PD deficiency patients show no symptoms until exposure to external triggers that could lead to moderate and severe symptoms, such as acute or chronic hemolytic anemia, favism, neonatal jaundice, and hyperbilirubinemia [4]. The hereditary G6PD enzyme deficiency distributes in males and varies among ethnic groups in different geographic regions, ranging from 0% in the native American to 20% or more in African and Asian [1,5]. Notably, the distribution of G6PD deficiency correlates with the malaria-endemic areas [6], showing the evolutionary selection of G6PD deficiency by malaria. The impairment of G6PD conferred the inhibition of in vitro parasite growth [7] and the protection against complicated malaria because of the early phagocytosis of infected RBC [8].
Previous clinical studies showed that G6PD A-variant in Africa protects against severe falciparum malaria disease only in hemizygous males [9] or heterozygous female children [10]. Although G6PD deficiency provides an advantage under natural selection by malaria, the patient with G6PD deficiency who received an anti-malarial drug, e.g., primaquine (PQ), may suffer hemolytic anemia [11]. The PQ prevents the relapse of P. vivax and P. ovale by killing the hypnozoite in liver cells and blocking the gametocyte of P. falciparum [12,13]. The severity of hemolysis depends on G6PD variants. Although the G6PD deficiency is rare, its prevalence in the regions where malaria is common would be over 10% [14]. Howes RE et al. [6] reported approximately 13–17% of Thai population carries common G6PD variants. This global prevalence of G6PD deficiency was estimated using a Bayesian geostatistical model which predicted the allele frequency of the G6PD deficiency map across endemic malaria countries. Among the Thai population, G6PD Mahidol, Viangchan, Canton, Kaiping, Mediterranean, Songklanagarind, Union, Vanua Lava, Chinese-5, Gaohe, Kerala-Kalyan and Quing Yan were observed. The G6PD variants are diverse among ethnicities and geographical regions. The Mahidol and Viangchan variants were the most common among Thais and people of other races. In the Thai population, G6PD Mahidol was as high as 94.92% of Thais living near the Thai-Myanmar border [15] while it was 20% in the north [16]. In contrast, Viangchan variant was dominant in southern Thailand with 46.8% found in Surat Thani province [17], 24.14% in Phuket province [18], and 31.3% in Songkhla province [19]. Among ethnic groups, G6PD Mahidol variant was detected in more than 90% of Karen and Myanmar people in the northwest [20], and 13.1% of Myanmar [17], and 37.9% of Moken people in the south [18]. In the Northeast, 31% of the Loa ethnicity carried the G6PD Viangchan variation [21]. In addition, Chinese G6PD mutations were found significant numbers in the northern region. Kaiping G6PD variant was found in 5.4% and 18%, and the Canton variant was 6.42% and 16% were presented in the Lue and Thai ethnic groups, respectively [16, 22].
However, G6PD deficiency testing in malaria patient is required before PQ administration, but the procedure is poorly implemented [11]. Information about the distribution of the G6PD variants would be necessary for the implication of primaquine policy. Even though several studies have reported the prevalence of G6PD deficiency in malaria patients from elsewhere in Thailand [23,24]. There is a limited data available for the southern Thailand, where malaria is still a problem and potential to increase. There has been no assessment of the G6PD deficiency frequencies in malaria-infected patients who resided or worked in malaria-endemic areas of southern Thailand. Therefore, the present study aims to investigate the prevalence of G6PD variants in malaria patients from southern Thailand.
MATERIALS AND METHODS
Ethical statement
This study was approved by the Ethical Review Committee for Research in Human Subjects, Prince of Songkla University (HSc-HREC-63-6-1-1).
Study area and subjects
This retrospective study was performed using dried blood spots samples from the previous study conducted in the southern Thailand [25–27], during 2012–2019. A total of 881 dried blood spots samples were diagnosed with malaria infection by the microscopic method from the malaria clinics at the Office of Disease Prevention and Control 11 and 12, Thailand. All finger-prick dried blood spot samples were collected on Whatman No.3 filter paper (GE Healthcare, Buckinghamshire, UK). These samples had been registered at malaria clinics from 5 sites in the Southern regions of Thailand: Ranong, Chumphon, Phang-nga, Surat Thani (located in the upper part of the south), and Yala (the southernmost province).
At the malaria clinic, microscopic examination of thick and thin smears was performed to detect infection, to estimate parasitemia and identify the parasite stage. The malaria parasites were counted against 200 white blood cells of thick film and 1,000 red blood cells of the thin film. Then, the parasite density (parasites/μl) was calculated from the number of parasites counted per 200 WBCs×8,000 cells/μl (assumed WBC count in patients) of thick film and parasitized cells×an estimated 5,000,000 average red cells divided by 20 fields×250 RBCs for thin blood smear [28]. The parasitic density was a geometric mean of 4,236 parasites/μl (95% CI, 8–47,607 parasites/μl (assuming WBCs (8,000/μl of blood). P. vivax infected individuals were treated with 25 mg/kg chloroquine and 0.25 mg/kg primaquine for 14 days as the first-line drugs according to the Ministry of Public Health, Thailand.
Plasmodium detection and G6PD variants analysis
Genomic DNA was extracted using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendation for dried blood spots. DNA was eluted in 100 μl of the elution buffer and used as templates for molecular approach. Plasmodium parasites species P. falciparum, P.vivax, P. ovale, and P. malariae were confirmed by nested PCR assay based on the 18S ribosomal RNA (18S rRNA) gene as described previously [29]. P. knowlesi species was confirmed with the cytochrome b (Cyt b) gene by nested PCR assay [30]. G6PD genotyping variants were identified using DiaPlexCTM G6PD Genotyping Kit (Asian type; SolGent, Korea) with one-step PCR. The different amplicon sizes from the 8 G6PD variants were produced as follow: Vanua Lava (383T>C, 154 bp), Mediterranean (563C>T, 262 bp), Coimbra (592C>T, 234 bp), Mahidol (4,87G>A, 337 bp), Viangchan (871 G>A, 501 bp), Kaiping (1,388G>A, 557 bp), Canton (1,376G>T, 681 bp) and Union (1,360C>T, 803 bp). Amplification was performed with an initial denaturation at 95°C for 15 min; 30 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 40 sec; and a final extension at 72°C for 5 min. PCR reactions were conducted in a 25 μl reaction mixture containing 5 μl of template, 12.5 μl of 2X multiplex PCR smart mix (G6PD Asian type), 2 μl of primer mixer (G6PD Asian type), 5.5 μl of nuclease-free water. The PCR products were resolved on 3% agarose gels electrophoresis at 100 V in the Tris-Borate-EDTA buffer and then visualized by UV light after staining with ethidium bromide. The internal control was confirmed at band 1,234 bp. G6PD Songklanagarind, the nucleotide changed from the TTC>ATC at codon 196 in the exon 4 of the G6PD gene, was detected by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). A pair of primers and PCR conditions were used as previously described [19]. According to the manufacturer’s instruction, the PCR products were digested with 10 U of FastDigest BstXI (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Data analysis
Malaria parasite density was determined in the G6PD deficient and non-deficient groups using the student’s t-test. The parasite count on a thick film was examined, consisting of 686/785 of P. vivax and 48/57 of P. falciparum and 8 samples were mixed infections between P. vivax and P. falciparum.
RESULTS
Prevalence of G6PD mutation variants
The study was conducted in Ranong (n=178), Chumphon (n=75), Phang-nga (n=7), Surat Thani (n=59) and Yala (n= 562). Among 881 malaria patients, 298 (33.9%) were females, and 506 (57.4%) were males, while no data was available for the rest 77 (8.7%). The majority of the patients were Thai (779; 88.4%), followed by Myanmar (99; 11.3%) and Laos (3; 0.4%), respectively (Fig. 1 and Table 1). P. vivax was detected in 785 (89.1%), P. falciparum in 61 (6.9%), P. knowlesi in 21 (3.1%), and mixed infection in 8 (0.9%) (P. vivax and P. falciparum) of the patients. The G6PD variants were detected in 26 out of 881 patients (2.9%) and the rest 855 patients were normal G6PD individuals (97.1%). The prevalence of 26 G6PD-deficient persons varied by the locations represented in Table 1. In order of prevalence from highest to lowest, they were 28.6% (2/7) in Phang-nga, 7.4% (13/178) in Ranong, 5.4% (4/75) in Phumphon 1.8% (1/59) in Surat Thani and 1.1% (6/562) from 1.1% (6/562) in Yala. These 26 samples had 5 different G6PD variants: Mahidol variant comprised 46.2% (12/26); Viangchan variant 42.3% (11/26), and a single case each of Kaiping, Union, and Mediterranean (3.85% of each). There was no Songklanagarind variant observed in this study. Among the G6PD deficiency individuals, 73.1% (19/26) were Thais, 23.1% (6/26) Burmese and 3.8% (1/26) Laos, while 13/26 (50%) were males and 8/26 (38.5%) females (Table 1). Only the samples infected with P. falciparum and P. vivax had G6PD variants, while P. knowlesi-infected blood samples possessed normal G6PD. As illustrated in Fig. 1, the prevalence and type of G6PD-deficiency varied among the regions: from 1.1% (6/562) in Yala to 7.3% (13/178) in Ranong. Ranong province at the Thai-Myanmar border showed a high frequency of the persons carrying G6PD variants, both in Thai and Burmese individuals with 53.8% (7/13) and 46.1% (6/13) respectively (Table 1). Among the Burmese ethnic group, 66.6% (4/6) of G6PD Mahidol and 33.4% (2/6) of G6PD Kaiping and Union, were observed respectively. G6PD mutations with a rate of 5.3% (4/75) were identified as Mahidol and Viangchan variants from Chumphon. Of these, 25% (1/4) was Mahidol variant observed in Lao, and the rest 75% (3/4) were Thais with 66.7% (2/3) of Mahidol and 33.3% (1/3) of Viangchan variants, respectively. One Thai patient with Mahidol variant, accounting for 1.7% (1/59), was found in Surat Thani. G871A mutation of G6PD Viangchan was observed in 2 out of 7 (28.6%) Thai patients from Phang-nga. In Yala province, 6 out of 562 samples (1.1%) were found to contain G6PD mutations, of which 66.7% (4/6) and 33.3% (2/6) were Mahidol and Viangchan variants, respectively. As described above, G6PD deficiency was relatively higher in the upper south provinces than in the lower part. Mahidol and Viangchan variants were the highest distribution in the upper south regions.
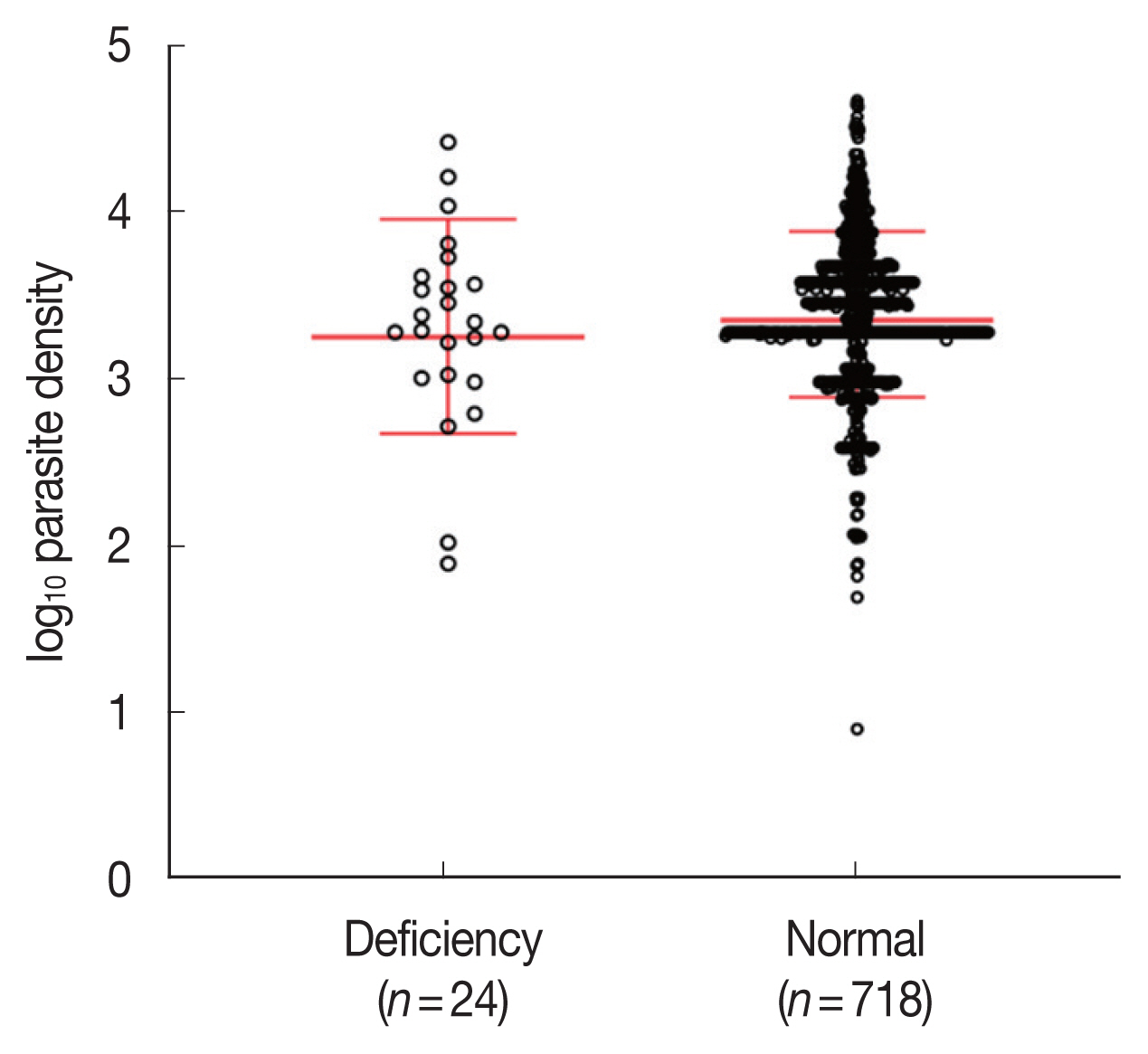
Associations between G6PD deficiency and parasitemia
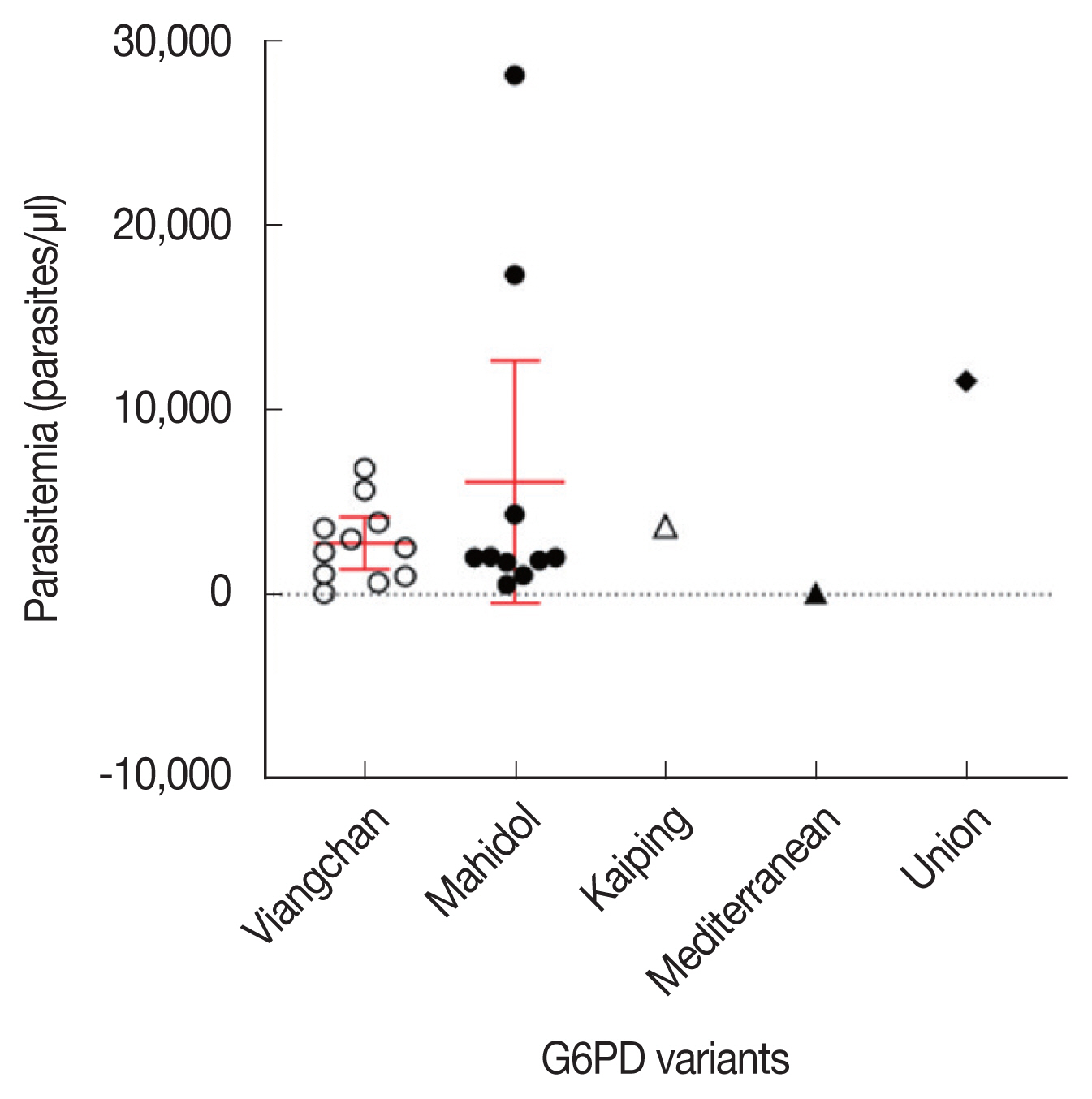
Parasite densities were compared among the G6PD-deficient (n=24) and G6PD-normal (n=718) samples. The average of parasite densities of G6PD-deficient and G6PD-normal samples were 4,456 (80–28,118 parasites/μl) and 4,141 (8–47,607 parasites/μl), respectively. There was no significant difference in parasitemia between G6PD-deficient and G6PD-normal groups (two-tailed unpaired t-test, P=0.539; Fig. 2). Fig. 3 describes the relationship between parasitemia and each of the variants of G6PD deficiency. The patients bearing the commonest variants, Viangchan and Mahidol, presented 2,738 parasites/μl (80–6,823 parasites/μl) and 6,098 parasites/μl (538–28,118 parasites/μl) in the blood. The parasitemia of the 3 individuals with Kaiping, Union, and Mediterranean variants was 3,701, 107, and 11,551 parasites/μl, respectively. In addition, the data showed that the mean of parasitemia of G6PD-deficient individuals infected with P. vivax was higher than that of P. falciparum infection, 4,717 parasites/μl (538–28,118 parasites/μl; n=19) versus 3,832 parasites/μl (80–11,551 parasites/μl; n=4), respectively.
DISCUSSION
The present retrospective cohort study investigates the prevalence of G6PD variants in malaria patients isolated from southern Thailand. The performance of G6PD screening before malaria treatment is uncommon in Thailand, which could impose the patients at risk of hemolysis. G6PD deficient populations were relatively more common in the upper provinces of southern Thailand than in the southernmost region. In Yala province where there were a higher number of malaria cases, the prevalence of the G6PD variant was relatively lower. According to Howes RE et al. [6], approximately 13–17% of the Thai population carries common G6PD variants. In this study, we analyzed the G6PD genotyping in only the southern region of Thailand. Twenty-six out of 881 malaria samples (2.9%) were identified as the G6PD mutation variants. The previous study, however, did not find G6PD variants in samples isolated from the southern region due to limited of the samples size, whereas the western, central and north-eastern regions presented G6PD variants in 5.36%, 14.28%, and 4.76% respectively [15]. Another study in the southern region revealed that 15.4% and 15.5% of healthy Phuket locals of Moken and Thai carried G6PD variants [23]. The lower prevalence of G6PD mutation variants observed in the present study might be due to the limitation of the DiaPlexC G6PD Genotyping Kit (Asian type) which is unable to detect the certain variants such as G6PD Quing Yuan, silent mutation, G6PD Gaohe [19], G6PD Namoru, G6PD Chatham and, G6PD Andalus [31,32].
World Health Organization has classified G6PD variants, based on the level of enzyme activity, into 5 classes from the most severe (Class I) to the mildest (Class IV): Class I, severe deficiency of the enzyme with chronic non-spherocytic hemolytic anemia; Class II severe deficiency with enzyme activity <10% of normal; Class III, moderate deficiency with enzyme activity 10–60% of normal; Class IV, very mild to none deficiency with enzyme activity 60–100% of normal; and Class V, increased enzyme activity [33]. Five types of G6PD mutation variants were identified in this study; Mahidol, Viangchan variants are associated with Class III variants, whereas Kaiping, Union, Mediterranean are associated with Class II variants [34]. However, clinical manifestations are an association between the phenotype and the genotype [35–38]. The low level of G6PD enzyme activity that is presented in G6PD mutations are risk of individuals hematologic changes such as hematocrit drops [39–41].
The Mahidol variant (487G>A) and G6PD Viangchan (871G> A) were the most predominant variants in our study. The previous studies also identified G6PD Mahidol and Viangchan variants as the major variants among healthy individuals in southern Thailand [17,18]. These variants were also commonly present in neighboring countries, including Cambodia, Laos, Myanmar [23,42]. We detected a small portion of G6PD Kaiping (1,388G>A), Union (1,360C>T), and Mediterranean (563C>T). Kaiping and Union variants were detected in the Burmese population. Kaiping variants were the most prevalent among the Chinese population in Southern China [43] and Malaysia [44]. It was also found at high frequency among Thai people of southern Thailand [30]. G6PD Mediterranean was commonly predominant in west Asia [5].
Our present study also showed that there was no difference in parasitemia between the G6PD-deficient and G6PD-normal patients. This was in agreement with the previous reports [20,45], which also suggested the absence of significant association between G6PD deficiency and parasite densities. P. vivax is preferentially infecting young red blood cells or reticulocytes, and hence, malaria parasites can replicate within these cells with the normal G6PD enzyme level [46]. A recent study has revealed that parasites produced their own G6PD enzyme to survive within the host red cell [47]. The other report suggests that the parasitized G6PD-deficient erythrocytes were susceptible to phagocytosis by monocytes, which could protect against malaria [48]. Although malaria cases in Thailand have significantly declined, the southern part of Thailand is still facing a high risk of malaria. The region shares 2 borders, with Malaysia in the south and Myanmar in the west, and the greater risk was related to high population mobility along the Myanmar-Thai border and social unrest in provinces bordering with Malaysia [13]. In recent years, P. vivax malaria cases have increased from 72% in 2016 to 83% in 2019, even though P. falciparum showed a reduction in patients [49]. P. vivax parasites are the main species causing severe illness and are relapsing. PQ with the daily dose of 0.25 mg/kg in 14-day regimen is prescribed to eliminate the dormant hypnozoites [11]. Prescribing PQ without G6PD testing has been reported to cause hemolysis in P. vivax infected patients, particularly at risk for the individuals with low G6PD enzyme activity. Even with the correct dose, intravascular hemolysis in male with G6PD Mahidol variant can occur [50]. It has been demonstrated that the G6PD Viangchan and Mahidol, the most common variants observed in southern Thailand, cause low enzymatic activity in RBCs and risk for acute hemolysis induced by PQ [51,52].
In this study, all dried blood samples were collected from malaria patients attending the malaria clinics. Before giving with Primaquine, the testing for G6PD deficiency is not available according to the national policy for malaria treatment. Hence, the measurement of G6PD enzyme activities is not generally considered at that time. In addition, the methods to determine the G6PD enzyme activity, such as spectrophotometry assay, cytochemical staining assay, fluorescent spot test, and point-of-care G6PD testing, are complex to perform in a field study. Hence, the lack of quantitative measurement of G6PD enzyme activity is a limitation of this study. However, the close relationship between G6PD variants and G6PD phenotypes has been established [35–38]. Further study is required to investigate the level of the G6PD enzyme activity associated with malaria-infected subjects. Therefore, our study highlights the importance of G6PD testing before PQ treatment to prevent hemolysis, raising awareness among healthcare policymakers and supporting the Thailand malaria elimination program.