Aly, Kim, Eraky, El Kholy, Ali, Miyoshi, and Omar: Evaluation of schistosomula lung antigen preparation and soluble egg antigen vaccines on experimental schistosomiasis mansoni
Abstract
Schistosomiasis causes significant morbidity and mortality worldwide. This study aimed to assess the effect of schistosomula lung antigen preparation (SLAP) and soluble egg antigen (SEA) on a murine schistosomiasis mansoni model. Ninety laboratory-bred male Swiss albino mice were divided into 6 groups. Two doses of the vaccine were given at 2-week intervals. All mice were subcutaneously infected with 80±10 Schistosoma mansoni cercariae 2 weeks after the last vaccination dose. They were sacrificed 7 weeks post-infection. Parasitological and histopathological studies were conducted to assess the effect of inoculated antigens (single or combined). The results showed that the combination of SLAP and SEA (combination group) led to a significant reduction in worm burden (65.56%), and liver and intestine egg count (59% and 60.59%, respectively). The oogram pattern revealed a reduction in immature and mature eggs (15±0.4 and 10±0.8, respectively) and an increased number of dead eggs in the combination group (P<0.001). In terms of histopathological changes, the combination group showed notably small compact fibrocellular egg granuloma and moderate fibrosis in the liver. A high percentage of destroyed ova was observed in the intestine of the combination group. This study demonstrates for the first time the prophylactic effect of combined SLAP and SEA vaccine. The vaccine induced a significant reduction in the parasitological and pathological impacts of schistosomiasis mansoni in hepatic and intestinal tissues, making it a promising vaccine candidate for controlling schistosomiasis.
Key words: Schistosomiasis, vaccine, SLAP, SEA
Introduction
Schistosomiasis is one of the most neglected tropical diseases associated with significant morbidity, mortality, and socioeconomic impact in many developing countries of Africa, Latin America, and Asia [ 1]. It is a public health concern in 51 endemic countries. Less than 50% of the 237 million people who required preventive chemotherapy in 2019 were treated [ 2]. In Africa, more than 112 and 54 million individuals were reported to have urogenital and intestinal schistosomiasis, respectively [ 3]. Praziquantel (PZQ), the only medication used to treat schistosomiasis at present, has been administered to large numbers of people to manage the disease over the past few decades. Because PZQ is inefficient against juvenile schistosomes and does not prevent re-infection, the prevalence and transmission of schistosomiasis have generally remained unabated despite the intense efforts of mass drug administration programs. Additionally, other efforts including controlling snail intermediate hosts and water sanitation and hygiene programs have had little to no effect. These problems show that the current control measures are completely ineffective at stopping transmission, necessitating the use of new control strategies [ 4]. Hence, novel vaccines and strategies for preventing schistosomiasis and reinfections are urgently needed [ 5]. Several studies have reported promising results of vaccines using either radiated cercariae or recombinant antigens [ 6]. A single-antigen vaccine is likely to elicit less protection against schistosomiasis than a multi-antigen vaccine [ 7]. The efficacy of a vaccine depends on the choice of parasite stage and use of an effective adjuvant [ 8]. Soluble egg antigen (SEA) acts as a potential anti-schistosome immunization candidate [ 9]. Schistosoma miracidium inside the ovum secretes glycoprotein antigens that pass through microscopic pores within the eggshell, hence the term SEA. These antigens elicit a vigorous immune response that encapsulates the ova in pre-granuloma collagen fibers and immune cells, predominantly eosinophils and macrophages. The granuloma formation presents a barrier to sequester egg toxicity and antigenicity [ 10]. The migrating schistosomulum stage, represented as schistosomula lung antigen preparation (SLAP), has been found to induce consistently high protective responses against Schistosoma infection in laboratory animals [ 11]. Although human vaccines against schistosomiasis are currently unavailable, strong evidence from human field studies and animal models supports the probability of developing vaccines for long-term protection [ 12]. No previous study used a combination of SLAP and SEA antigens as a vaccine candidate. Thus, this study aimed to assess the effect of SLAP and SEA on a murine model of schistosomiasis.
Materials and Methods
Parasite
Schistosoma mansoni cercariae were obtained from infected Biomphalaria alexandrina snails, which were purchased from the Schistosome Biological Supply Unit, Theodor Bilharz Research Institute, Giza, Egypt, and maintained in the laboratory according to the guidelines proposed by Becker and Lamprecht [ 13]. Schistosoma mansoni cercariae from the snails (after exposure to light for at least 4 h) were used to infect the mice through the subcutaneous route.
Experimental animals
Laboratory-bred male Swiss albino mice weighing nearly 20–30 g were used in this study. All animal-related procedures in this study were in accordance with the International Guiding Principles for Biomedical Research, as issued by the International Organizations of Medical Science [ 14]. The study was revised and approved by the Institutional Animal Care and Use Committee, Medical Ethics Committee of the Faculty of Medicine, Benha University (approval no. MD 1-8-2021).
Antigen preparation
SLAP preparation: Schistosomula were extracted using the perfusion technique from pulmonary tissue 7 days post-infection. The lungs were placed in a small beaker with 2 ml of RPMI 1640 medium (Sigma, St. Louis, MO, USA) with penicillin and streptomycin and finely chopped to allow the schistosomula to migrate out of the capillary beds. The suspension was placed in a 50-ml centrifuge tube, incubated for 3 h at 37°C, and then centrifuged at 100×g at 4°C. Thereafter, the supernatant was removed. The last pellet was added to 2–3 ml of RPMI 1640/penicillin/streptomycin in a petri dish with a diameter of 60 and 15 mm. The number of schistosomula in the total dish was counted using a dissecting microscope. The schistosomula were gathered using a fine-tip Pasteur pipette and transferred into culture jars for testing [ 15]. SEA preparation: Eggs were collected from liver tissues and suspended in 4°C PBS at a concentration of 100,000 eggs/ml. The eggs were homogenized on ice using a pre-chilled homogenizer and tight pestle. The crude mixture was centrifuged at 4°C at 200×g for 20 min. The supernatant was collected and centrifuged for 90 min at 100,000×g at 4°C. The supernatant was sterilized by passing it through a 0.2-μm filter [ 16]. The protein content of prepared antigens was determined according to Bradford’s method [ 17]. The antigens were added with Complete Freund’s Adjuvant (CFA) (Sigma) and emulsified in phosphate-buffered saline at a ratio of 2:1.
Experimental design
Six groups of Swiss albino mice (15 mice in each group) were classified as follows: group I, non-infected and non-immunized (healthy control group); group II, infected and non-immunized (infected control group); group III, received CFA (50 μl); group IV, immunized with SLAP (50 μl SLAP+50 μl CFA); group V, immunized with SEA (100 μl SEA+50 μl CFA); and group VI, immunized with SLAP and SEA (combination group; 50 μl CFA+25 μl SLAP +50 μl SEA).
Each mouse received a single subcutaneous injection of vaccine. Then, a booster dose of vaccine was given 2 weeks later. Two weeks after the last dose of vaccine, all mice were infected with 80±10 S. mansoni cercariae, which were injected subcutaneously in the abdomen [ 18]. The mice were sacrificed 7 weeks post-infection through rapid decapitation, and parasitological and histopathological studies were conducted to assess the effect of antigens.
Evaluation of the vaccine efficacy
Parasitological studies
Worm distribution: Perfusion of sacrificed mice to detect adult worm distribution. The principle of this technique is to isolate the region to be examined and perfuse the vessels in which the worms may be present. The needle was connected to the automatic pumping machine and inserted in the inferior vena cava. Saline was pumped from the machine through the inferior vena cava to the liver. The worms were washed out and came out of the cut portal vein. Then, they were collected and counted [19]. Tissue egg counts: Pieces of the liver and intestine were removed from each animal after perfusion to determine the number of eggs/gram of tissue. The gut was slightly dilated and cleaned with saline to remove any fecal waste that was in the lumen. Ten milliliters of 5% potassium hydroxide solution was used for each piece of liver or intestine after it had been dried on filter paper, weighed, and deposited separately. The tissues were hydrolyzed after 24 h of incubation at 37°C. Three samples (0.1 ml each) were pipetted from the digest and placed on a counting slide. After they had been agitated vigorously on a magnetic mixer for 1 min, they were analyzed. The number of ova in each tissue was counted, and the average number was counted [20]. Oogram pattern: The oogram pattern was used to evaluate the degree of ova maturity and viability, which may reflect the vaccine’s effect on oviposition and maturation [21]. The oogram showed the developmental stages of ova (immature, mature, and dead) in the small intestine of experimental animals.
Histopathological studies
Sections were stained with hematoxylin and eosin [ 22] or Masson’s trichrome stain to demonstrate collagen fibers and measure granuloma dimensions easily. The liver sections were histopathologically examined for the presence of Schistosoma ova or granuloma, and their diameter and number were counted in each group. Intestine sections were examined for the presence or absence of S. mansoni ova, granuloma, any villous abnormality, and intensity of inflammatory cellular reaction.
Statistical analysis
Data were analyzed by SPSS program (version 20; SPSS Inc., Chicago, IL, USA). Quantitative data were analyzed using mean and standard deviation. Reduction % (R%)=(No. of worms in the infected non-treated control group–No. of worms in the infected treated group ×100)/(No. of worms in the infected non-treated control group) [ 23]. Differences among the studied groups were estimated by one-way analysis of variance, and the Tukey–Kramer test was used as the post hoc test. Statistical significance was considered at P≤0.05.
Results
Parasitological assessment
The vaccine’s effect on worm burden is shown in Table 1 and Fig. 1. Group VI (combination group) was the most affected group, in which the reduction rate of the worm burden was statistically significant (65.5%), followed by groups IV and V with significant reduction rates of 28.63% and 17.57%, respectively ( P<0.01). In terms of the mean egg count (the mean number of eggs/gram of tissue) in the liver and intestine, group VI showed a significant reduction rate (59.0% and 60.59%, respectively) followed by groups IV (45.2% and 35.03%, respectively) and V (40.3% and 21.9%, respectively) ( Table 1; Fig. 2). The oogram pattern showed that group VI presented a statistically significant reduction of the mean number of immature and mature ova (15±0.4 and 10±0.8, respectively) followed by groups IV (35.4±1.2 and 27±1.1, respectively) and V (38.2±1.43 and 29±1.0, respectively). Additionally, group VI showed a statistically significant increase in the mean number of dead ova (50.5±4.33), followed by groups IV and V (30.2±3.0 and 20.0±6.0, respectively) ( Table 2).
Histopathological results
Table 2 and Fig. 3 show that group VI had a significant reduction rate of granuloma number and diameter (85% and 72.8%, respectively), followed by groups VI (60% and 53%, respectively) and V (40% and 45%, respectively). The histopathological study of the liver stained by Masson’s trichrome stain is shown in Fig. 4.
Group I is showing deposition of fibrous tissue. Group II has mild fibrosis around the granuloma (+). Group III has marked fibrosis (+++) around the granuloma. Vaccinated groups (IV, V, and VI) have moderate fibrosis (++) around the granuloma. Regarding hematoxylin and eosin stains of the intestine ( Fig. 5), the intestine sections of group I showed normal villous (left) and crypt (right) patterns. All infected groups showed viable ova with intact miracidium and acute inflammatory cellular reaction around Schistosoma ova, with a high percentage of eosinophils. Group VI showed mostly destroyed ova.
Discussion
Identifying vaccine targets is difficult because of the complexity of the schistosome’s life cycle. Many experimental trials to develop an effective human vaccine for schistosomiasis have been reported. More than 100 schistosome vaccine antigens have demonstrated some degree of protection in murine schistosomiasis models. A vaccine would lessen schistosomiasis morbidity by inducing immune responses that would decrease the parasite load and diminish egg production [ 24]. The Schistosoma parasite secretes and excretes various antigens into the host’s bloodstream; these antigens are divided into cercarial, adult worm, and egg antigen categories depending on the state of the parasite’s development [ 25]. Many laboratories have isolated different antigens from crude SEA and soluble worm antigen preparation (SWAP). A previous study has shown that antigens derived from various phases of the parasite’s life can trigger a protective reaction in mice [ 26]. Studies on the preventive and anti-fecundity effects of Sm-p80 have shown that combining different antigens can result in higher levels of vaccine-induced protection. For example, an adjuvant, synthetic hexa-acylated lipid A derivative (glucopyranosyl lipid A) formed in aluminum has been combined with recombinant Sm-p80 to increase the vaccine’s efficacy. The biological functions of schistosomes such as schistosomula migration and fecundity represent key potential sites to target parasites for elimination through vaccination [ 27]. During the acute stage, the predominant immune response to schistosomula antigens and as the disease progresses, the body response is caused by egg antigens [ 28]. Therefore, this research assessed the prophylactic impact of SLAP combined with SEA as a new vaccine candidate on a murine model of schistosomiasis. The results in the present study showed variable degrees of reduction in worm burden. A significant reduction in worm burden was observed in the combination group (65.56%). Many previous reports showed variable degrees of reduction in worm burden in vaccinated groups [ 29]. The variation between their data and our data may be a result of using different antigen doses and schistosomula age as schistosomula were extracted from the lungs 18 days post-infection. Egg reduction is more influential in vaccine efficacy than the reduction of adult worms as a reduction in hepatic egg count reflects a decrease in eggs entrapped in the liver, leading to improvement in liver pathology, reduction in intestinal egg count, and decreased transmission rate and pathology of the parasite [ 30]. The combination of SLAP and SEA resulted in a significant reduction in egg count per gram of liver and intestine tissues (59% and 60.59%, respectively). Our results were in accordance with those of Etewa et al. [ 9], who observed a significant reduction of S. mansoni eggs in the liver. Lower values were obtained by El-Ahwany et al. [ 29], with 42.8% and 8.4% reduction of eggs in the liver and tissue, respectively. The reduction of the immature and mature egg count in vaccinated groups goes in parallel with the significant reduction in the adult count recorded in the present study. These changes could be explained by the cessation of oviposition because of the death of adult worms or the reduction of female fecundity because of the decoupling effect [ 31]. In the present study, different groups had a reduction in granuloma count and granuloma diameter, with the combination group having the most significant reduction (85% and 72.8%, respectively). These results reflect that the reduction of granuloma number might be related to a decrease in tissue egg count rather than the inability of eggs to initiate granuloma formation. The granulomatous reaction may be attributed to SEA-specific stimulation of immune reaction against Schistosoma eggs in hepatic pathology. These results were consistent with those of other studies [ 32], indicating that the vaccine could induce a partial reduction in worm burden and decrease the granuloma number and diameter, which were attributed to an increase in CD8+ cells in immunized groups and a reduction of CD4+ cells. Owing to the downregulation of immune response, newly formed granulomas are smaller and contain killed eggs; meanwhile, granulomas that are formed earlier gradually resolve to be replaced by fibrous tissue, leading to the amelioration of symptom severity [ 33]. Fibrosis is the excessive accumulation of fibrous connective tissue (e.g., collagen and fibronectin) in and around inflamed or damaged tissue [ 34]. This indicates a good protective immune response to the combined vaccine in our study. In Etewa et al.’s study [ 9], the liver sections of the SEA-vaccinated group had fewer numbers and smaller sizes of granuloma; degenerated ova surrounded by empty spaces were enclosed in fibrocellular granulomas in addition to mild lobular inflammation. In the present study, no statistical difference was observed between the group receiving CFA adjuvant (III) and the infected non-immunized group (II). In addition, the SLAP-immunized group (IV) obtained better results than the SEA-immunized group (V). The administration of SLAP and SEA (VI) prior to infection resulted in decreased worm load and hepatic and intestinal ova and changes in the oogram pattern. This could be due to the augmentation of immune response or a result of primary infection. According to Etewa et al. [ 9], SEA is preferable than SWAP. The ovum’s Schistosoma miracidium (SEA) secretes glycoprotein antigens, which pass through tiny openings in the eggshell. A strong immune reaction to these antigens surrounds the ova in pre-granuloma collagen fibers and immune cells, primarily eosinophils and macrophages. Granuloma formation is a barrier to sequestering egg toxicity and antigenicity. Moreover, proteins were identified from S. mansoni SLAP, which can stimulate protective Th1 cell-mediated immune responses [ 35]. A purified SLAP preparation causes a significant decrease in worm burden, egg load, granuloma diameter, and collagen content and increase in the proportion of degenerated ova. Moreover, it can improve pathological alterations in pulmonary and hepatic tissues [ 29]. However, which of these immune responses is directly involved in worm-killing and protective immunity remains unknown. Further studies are needed to assess the effect of our vaccine on Th1 and Th2. An effective anti-schistosome vaccine would greatly decrease the morbidity associated with schistosomiasis via protective immune responses, leading to reduced worm burden and decreased egg production and its pathology [ 24]. The combined vaccine apparently induced an improved immune response against antigenic components. In conclusion, the obtained parasitological and histopathological findings highlighted the protective response of the combined vaccine (SLAP and SEA). The combination of SEA and SLAP antigens induced a marked decrease in worm burden, egg load, and granuloma count and diameter and an improvement of pathological changes in hepatic and intestine tissue.
Acknowledgments
This study was partially supported by a grant from the Program of the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID, JP22wm0125004) from the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT), and the Japan Agency for Medical Research and Development (AMED).
We are deeply grateful to Prof. Tarek Aboushousha, Department of Pathology, Theodor Bilharz Research Institute, for his important role in performing the histopathology of this study.
Notes
Author contributions
Conceptualization: Aly N, Eraky MA, El kholy A, Omar RE
Data curation: Ali B
Formal analysis: Aly N, Eraky MA, El kholy A, Omar RE
Funding acquisition: Miyoshi SI
Investigation: Ali B
Methodology: Ali B, Omar RE
Resources: Ali B, Miyoshi SI
Software: Aly N, Kim HS
Supervision: Aly N, Eraky MA, El kholy A, Omar RE
Validation: Aly N, Kim HS, Eraky MA
Visualization: Ali B
Writing – original draft: Aly N, Omar RE
Writing – review & editing: Aly N, Kim HS, Eraky MA, El kholy A, Miyoshi SI
Conflict of interest
The authors have declared that no competing interests exist.
References
4. Campbell SJ, Biritwum NK, Woods G, Velleman Y, Fleming F, et al. Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted Helminthiasis and Schistosomiasis control. Trends Parasitol 2018;34(1):53-63
https://doi.org/10.1016/j.pt.2017.09.004
  7. Ewaisha RE, Bahey-EI-Din M, Mossallam SF, Amer EL, Aboushleib HM, et al. Combination of the schistosomal antigens Sm14 and Sm29 elicits significant protection against experimental Schistosoma mansoni infection. Exp Parasitol 2014;145:51-60
https://doi.org/10.1186/s12879-015-0906-z
   8. Araujo JM, de Melo TT, de Sena IC, Alves CC, Araujo N, et al.
Schistosoma mansoni schistosomula tegument (Smteg) immunization in absence of adjuvant induce IL-10 production by CD4+ cells and failed to protect mice against challenge infection. Acta Trop 2012;124:140-146
https://doi.org/10.1371/journal.pone.0160118
   9. Etewa S, AL-Hoot AAA, Sharaf HM, Moawad HS, Mohamed SM, et al. Modelling approaches to predict and evaluate schistosomiasis immunization utilizing SEA loaded on chitosan nanoparticles via liver tissue differentiation and angiogenesis. Parasitol United J 2019;12:197-208
https://doi.org/10.21608/puj.2019.16532.1051
 13. Becker W, Lamprecht I. Microcalorimetric investigations of the behavior and infectivity of miracidia of Biomphalaria glabrata and Schistosoma mansoni
. Z Parasitenkd 1977;53(3):297-305 (in German). https://doi.org/10.1007/BF00389947
  17. Bradford MM. A rapid and sensitive method for the quantitation of microgram qualities of protein utilizing the principle of protein dye binding. Anal Biochem 1976;72:248-254
https://doi.org/10.1016/0003-2697(76)90527-3
  18. Peters AP, Warren KS. A rapid method of infecting mice and other laboratory animals with S. Mansoni subcutaneous infection. J Parasitol 1969;55(3):558
https://doi.org/10.2307/3277297
 20. Cheever AW. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull World Health Organ 1968;39(2):328-331.   21. Pellegrino J, Oliveira CA, Faria J, Cunha AS. New approach to the screening of drugs in experimental schisosomaiasis mansoni. Am J Trop Med Hyg 1962;11:201-215
https://doi.org/10.4269/ajtmh.1962.11.201
  25. Van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop 2000;77(1):69-80
https://doi.org/10.1016/s0001-706x(00)00115-7
  26. Teixeira de Melo T, Michel de Araujo J, Do Valle Durães F, Caliari MV, Oliveira SC, et al. Immunization with newly transformed Schistosoma mansoni schistosomula tegument elicits tegument damage, reduction in egg and parasite burden. Parasite Immunol 2010;32(11–12):749-759
https://doi.org/10.1111/j.1365-3024.2010.01244.x
  29. El-Ahwany E, Bauiomy IR, Nagy F, Zalat R, Mahmoud O, et al. Regulatory cell responses to immunization with a soluble egg antigen in Schistosoma mansoni infected mice. Korean J Parasitol 2012;50(1):29-35
https://doi.org/10.3347/kjp.2012.50.1.29
   30. Ranasinghe SL, Duke M, Harvie M, McManus DP. Kunitz-type protease inhibitor as a vaccine candidate against schistosomiasis mansoni
. Int J Infect Dis 2018;66:26-32
https://doi.org/10.1016/j.ijid.2017.10.024
  31. Mati VL, Melo AL. Current applications of oogram methodology in experimental schistosomiasis; fecundity of female Schistosoma mansoni and egg release in the intestine of AKR/J mice following immunomodulatory treatment with pentoxifylline. J Helminthol 2013;87(1):115-24
https://doi.org/10.1017/S0022149X12000144
  32. Pinho JMR, Cardoso FC, Lopes DO, Pinheiro CS, Caliari MV. Immunization with SmIg, a novel tegument protein from Schistosoma mansoni, fails to induce protection in mice but reduces liver pathology. Parasitology 2010;137(7):1079-1088
https://doi.org/10.1017/S0031182009991387
 
Fig. 1
Mean reduction rate of worm burden. Group I, non-infected non-immunized (Healthy control group); Group II, infected non-immunized; Group III, received Complete Freund’s adjuvant (CFA); Group IV, immunized with SLAP; Group V, immunized with SEA; Group VI, immunized with SLAP and SEA (combination group). 
Fig. 2
Mean reduction rate of egg count per gram of liver and intestine tissues. Experimental conditions are indicated in the legend of Fig. 1. 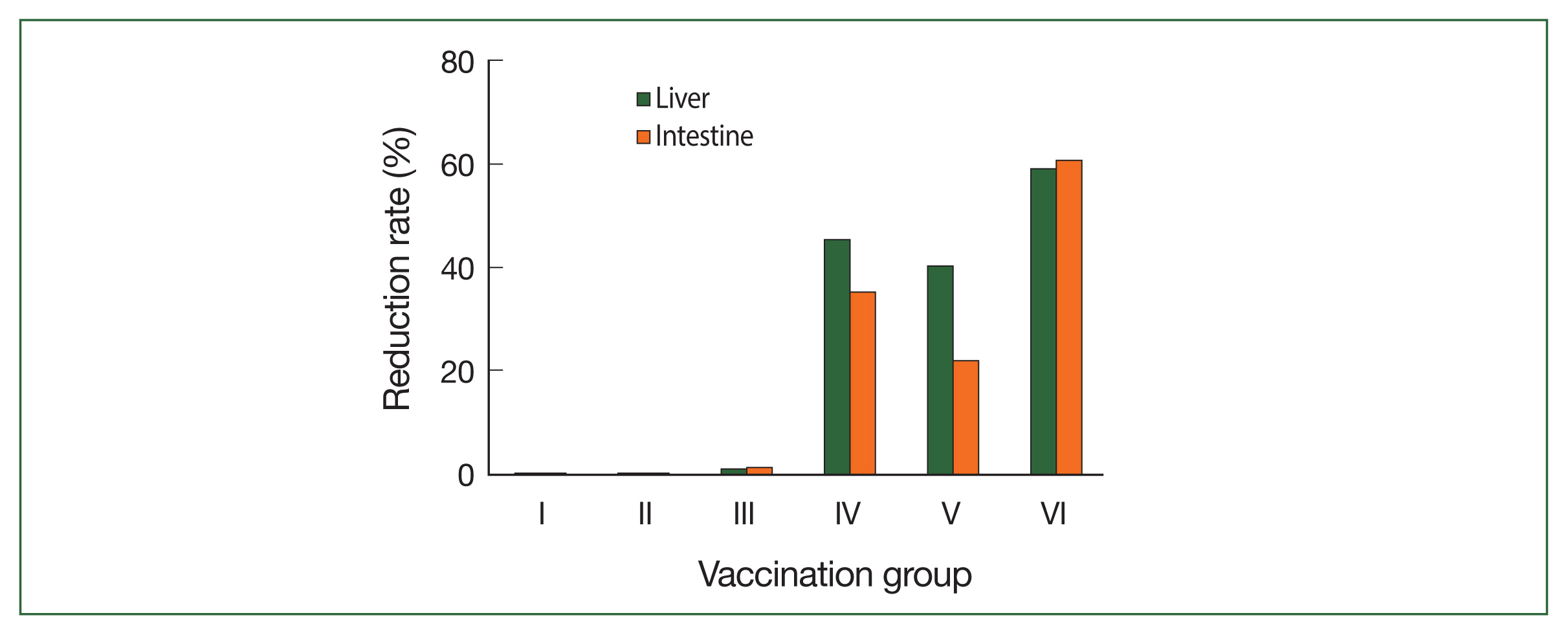
Fig. 3
Histopathological study of the liver stained with hematoxylin and eosin stain (×200). (A) Normal hepatic lobular architecture. Bar=100 μm. (B) Cellular egg granuloma with intact central ovum (black arrow). (C–E) Egg granulomas with degenerated central ova (black arrows). (F) Fibrocellular egg granuloma with regular outline and degenerated central ovum (black arrow). 
Fig. 4
Histopathological examination of the liver stained with Masson’s trichrome stain (×200). Bar=100 μm. (A) Deposition of fibrous tissue. (B) Mild fibrosis around the granuloma (+) (black arrow). (C) Marked fibrosis (+++) around the granuloma. (D–F) Moderate fibrosis (++) around the granuloma. 
Fig. 5
Histopathological study of the intestine stained with hematoxylin and eosin (×400). Experimental conditions are indicated in the legend of Fig. 1. (A) Group I mice showing normal villus (black arrow). Bar=100 μm. (B) Group II mice showing many S. mansoni ova within the mucosa (black arrow). Bar=50 μm. (C) Group III mice showing S. mansoni ovum surrounded by a large number of inflammatory cells with a high percentage of eosinophils (yellow arrows). Bar=50 μm. (D) Group IV mice showing many S. mansoni ova within the mucosa with some intact (red arrow) and degenerated (yellow arrow) miracidia. Bar=50 μm. (E) Group V mice showing many S. mansoni ova within the mucosa (yellow arrow). Bar=50 μm. (F) Group VI mice showing many S. mansoni ova within the mucosa with mostly destroyed ova (yellow arrows). Bar=50 μm. 
Table 1
Mean worm burden and egg count in different groups
|
Groups |
Worm burden |
Egg count per gram of liver |
Egg count per gram of intestine |
|
No. |
Mean±SD |
R (%) |
Mean±SD |
R (%) |
Mean±SD |
R (%) |
|
I |
- |
- |
- |
- |
- |
- |
|
II |
17.2±2.9 |
- |
2,548.1±225.1 |
- |
2,780.0±170.8 |
- |
|
III |
17.1±2.6 |
0.6 |
2,525.2±221.4 |
0.9 |
2,750.2±140.9 |
1.1 |
|
IV |
11.0±1.3 |
28.6*
|
1,755.0±164.6 |
45.2*
|
2,100.3±68.0 |
35.0*
|
|
V |
14.2±1.4 |
17.6*
|
1,922.1±124.2 |
40.3*
|
2,222.1±73.6 |
21.9*
|
|
VI |
6.8±0.9 |
65.6*
|
1,044.7±103.7 |
59.0*
|
1,262.7±63.3 |
60.6*
|
Table 2
Oogram pattern and granuloma parameters (number and diameter) in different groups
|
Group |
Oogram pattern |
Granuloma number |
Granuloma diameter |
|
No. |
Mean±SD |
Mean±SD |
Mean±SD |
Mean±SD |
R (%) |
Mean±SD |
R (%)
Mean±SD |
|
I |
- |
- |
- |
- |
- |
- |
- |
|
II |
45.2±1.8 |
50.2±1.66 |
4.7±1.9 |
10.0±2.0 |
- |
184.0±22.0 |
- |
|
III |
45.0±1.7 |
49.1±1.5 |
4.8±2.1 |
9.0±2.41 |
10.0 |
170.0±56.16 |
7.6 |
|
IV |
35.4±1.2*
|
27.0±1.1a
|
30.2±3.0a
|
4.0±0.7 |
60.0*
|
85.0±19.6 |
53.8*
|
|
V |
38.2±1.4*
|
29.0±1.0a
|
20.0±6.0*
|
6.1±3.6 |
4.0*
|
100.0±34.1 |
45.0*
|
|
VI |
15.0±0.4*
|
10.0±0.8a
|
50.5±4.33b
|
1.5±6.7 |
85.0*
|
50.0±6.5 |
72.8*
|
|
|











