AbstractPaleoparasitology is a discipline that applies existing conventional and molecular techniques to study parasites found in ancient ruins. This review focuses on the history of the discovery of parasites (mostly helminth eggs and larvae) in archaeological soil samples and mummies in Korea from the Three Kingdoms Period to the Joseon Dynasty (100 BCE-1910 CE). We also briefly review important milestones in global paleoparasitology. The helminth species reported so far in Korea included Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis (larva), Trichostrongylus sp. (larva), Paracapillaria philippinensis (syn. Capillaria philippinensis), Enterobius vermicularis, Fasciola hepatica, dicrocoeliids, Paragonimus westermani, Clonorchis sinensis, Metagonimus yokogawai, Pygidiopsis summa, Gymnophalloides seoi, Isthmiophora hortensis, Dibothriocephalus nihonkaiensis (syn. Diphyllobothrium nihonkaiense), and Taenia spp. tapeworms. The findings obtained by Korean paleoparasitologists/archaeologists have brought about deep insight into the status of helminthic infections in Korea’s past populations. Continued paleoparasitological research is essential for further understanding of ancient parasites and parasitic diseases in Korea.
IntroductionPaleoparasitology (or archaeoparasitology) and paleopathology, in the sense of using a systematic approach, began around 1910 by Dr. Ruffer MA in Egypt [1]. Schistosoma haematobium calcified eggs were identified and described under the name Bilharzia haematobia from the histopathological sections of the kidneys of 2 Egyptian mummies dated to 1250-1000 BCE (Before the Christian Era) [1]. In 1944, Szidat [2] found Ascaris lumbricoides and Trichuris trichiura eggs from the intestinal content of 2 Prussian (northern part of Germany) bog bodies, a man, and a woman, together with possible eggs of Dibothriocephalus latus (syn. Diphyllobothrium latum) and mite eggs in the man. Since then, over the succeeding several decades, paleoparasitology has attained remarkable progress and development worldwide [3]. Experts in this research field have examined soil sediments, strata soil, coprolites, and internal organs and tissues of mummies excavated from archaeological sites and tombs and discovered ancient helminth eggs, larvae, and their DNAs using conventional and molecular techniques [3].
In Korea, paleoparasitology research began in the late 1990s using archaeological wetland soil samples from Shinchang-dong, Gwangju Metropolitan City (Gwangju) (Fig. 1, no. 1) [4]. Helminth eggs, including A. lumbricoides and T. trichiura, were detected in samples from 100 BCE (Three Kingdoms period, Baekje Dynasty) [4]. The second report, published in 2003, was on the discovery of A. lumbricoides, T. trichiura, and Clonorchis sinensis eggs in pit soil specimens from archaeological sites in Chilgok-gun, Daegu Metropolitan City (Daegu) (Fig. 1, no. 2), dating to 668–935 CE (the Christian Era) (Unified Silla Dynasty), and of the eggs of 2 unknown trematode species in soil samples from Uljin-gun, Gyeongsangbuk-do (Province), dating to 100 BCE (Three Kingdoms period, Silla Dynasty) [5]. Since then, soil sediment, precipitate, strata soil (Fig. 2A), and pit soil from ancient places, like Seoul, Buyeo, and Gyeongju have been investigated for helminth eggs and larvae.
In 2007, studies began to use human mummy specimens (Fig. 2B), mostly from the Joseon Dynasty (1392–1910) [6–8], which became a big achievement in paleoparasitological research in Korea. The medieval Korean mummies are superb in their status of preservation and are highly useful for paleopathological and paleoparasitological purposes [3]. The tombs in the Joseon Dynasty were built using lime, sand, and red clay filled around the coffin which became hardened like a stone after a long while, forming a layer of the lime soil mixture barrier (LSMB), and the coffin could be protected from the possible intrusions of insects and other biological or physical invaders [9]. With the help of archaeologists, the mummy studies greatly enabled paleoparasitologists in Korea to achieve meaningful progress in understanding the status of ancient parasites and parasitic diseases in medieval Korean populations [9]. Another big step forward is probably the use of molecular techniques, including DNA analysis and gene sequencing, on archaeological samples obtained in Korea. Ancient DNAs (aDNAs) of Ascaris sp. [10], T. trichiura [11], and C. sinensis [12] were successfully extracted and sequenced from organic materials from mummy and soil sediments in medieval tombs of the Joseon Dynasty. Since then, plenty of useful research reports, including conventional and molecular approaches, have been gathered.
This review briefly summarizes the 26-year history of ancient parasite research in Korea, with special paleoparasitological consideration of each helminth species discovered. Important milestones in global paleoparasitology research are also briefly reviewed, including major achievements on some currently undetected helminth species in Korea.
Ascaris lumbricoidesGeneral views
A. lumbricoides (Nematoda: Ascaridae) is the largest nematode among those infecting humans and the representative species of soil-transmitted helminths [13]. It is distributed worldwide. Its eggs are excreted in human feces and develop into embryonated eggs, the infective stage to humans, mostly in soil [13]. The mode of infection is the accidental ingestion of soil, vegetables, and fruits contaminated with embryonated eggs. This parasite may cause variable clinical symptoms, including indigestion, diarrhea, abdominal cramp, and growth retardation [13]. Two types of A. lumbricoides eggs are produced, i.e., unfertilized eggs and fertilized eggs, and only the fertilized eggs undergo development into larvae and are responsible for transmission to new host individuals [13]. Ascaris suum, the pig Ascaris, is a closely related species, both morphologically and phylogenetically, and the possibility of human A. suum infection has been suggested [13].
Paleoparasitological studies on Ascaris spp. in KoreaOne of the archaeological materials frequently examined is the soil (including strata soil), soil sediment, or soil precipitate in an environment separated from tombs. Such a site may include an ancient toilet or a disposal place for human and/or animal excreta. If Ascaris sp. eggs were found in soil specimens sampled from a pit or soil strata, which probably had a connection with toilets or latrines for humans, those were regarded as the human roundworm, A. lumbricoides [5,14]. On the other hand, eggs with the same morphology from places remote from human toilets were assigned to A. suum or Ascaris sp., if supported by a molecular study [10,15,16]. However, it should be noted that the longtime debates on the taxonomy of human and pig Ascaris, i.e., A. lumbricoides and A. suum, have been almost solved recently through extensive molecular studies [17]. The two species appeared to be hardly distinguished by morphological and molecular means, and it has been proposed to treat A. suum as a synonym of A. lumbricoides [17]. We followed this proposal, although in Table 1 we tried to keep the original diagnosis (A. lumbricoides or Ascaris sp.) given by the authors of each study. Notably, Korean medieval tombs with mummies inside are usually encapsulated by a stone-like solid layer of LSMB and were separated from the surroundings [18]. Thus, the eggs of Ascaris detected in the feces or coprolites of the mummy’s intestines or soil sediment near the hip bones or sacrum were assigned as those of human origin, A. lumbricoides.
In Korea, the detection of A. lumbricoides eggs in archaeological samples was reported for the first time by Kwangju National Museum in 1997 (Table 1) [4]. The material examined was wetland soil in Shinchang-dong, Gwangju dating to the Early Iron Age (100 BCE, Baekje Dynasty). The next study was the examination of the archaeological soil in Chilgok area, Daegu in 2003, dating to the Unified Silla period (668–935 CE), where A. lumbricoides eggs (fertilized, 55.0±3.7 μm long and 49.4±2.2 μm wide, and unfertilized, 80.0 μm long and 50.0 μm wide) were found [5]. Since then, at least 25 studies (including the above 2) have been published (Table 1) [4–6,10,14–16,18–35].
Soil studiesAfter the two pioneering studies [4,5], a few A. lumbricoides eggs together with a great number of T. trichiura eggs were discovered in soil samples from the ruins of a moat encircling the royal palace at Gyeongju-1 (Fig. 1, no. 5) of the Silla Dynasty dating to the 5th–8th century [19]. Regarding the smaller number of A. lumbricoides eggs, in comparison with a great number of T. trichiura eggs, it was speculated that it might have been due to an easier and faster decay of A. lumbricoides eggs than T. trichiura eggs in certain ancient samples [19]. In this regard, it is noteworthy that Ascaris aDNA was detected from human coprolites of pre-Columbian South American times even in the microscopic absence of the eggs, through the sequencing analysis of cytochrome b gene [36]. However, in two Korean studies in which the eggs of A. lumbricoides (Fig. 3A, B) were detected in strata soil (old palace, old road, and old arsenal) and streambed soil (alley, side gutter, etc.) from old Capital City, Seoul (Fig. 1, no. 10) dating to the Joseon Dynasty (14th–19th century), the number of A. lumbricoides eggs was not so small compared to T. trichuris eggs [22,24]. Moreover, in soil samples obtained from V-shaped pits (presumed to be toilets) at Buyeo-1 (Fig. 1, no. 15) [14] and geological strata [27] at Buyeo-2 (Fig. 1, no. 16) dating to the ancient Baekje Dynasty (6th–7th century), the number of A. lumbricoides eggs was rather greater than that of T. trichiura eggs. Similarly, in soil sediment samples from Gimhae (Fig. 1, no. 17) and Changnyeong (Fig. 1, no. 18) dating to ~ 676 CE of the ancient Silla Dynasty [28], Seoul dating to the 15th century of the Joseon Dynasty [29], and Buyeo-2 and Buyeo-3 (Fig. 1, no. 27) dating to the 5th–7th century of the Baekje period [32,34], A. lumbricoides and T. trichiura eggs were detected almost equally in their amount. Based on these reports, the relative difference in the decaying time of different helminth species eggs [19] needs further verification.
Mummy studiesMummy studies reporting A. lumbricoides eggs were first published in 2007 in a child mummy from Yangju (Fig. 1, no. 3) dating to the 17th century of the Joseon Dynasty [6]. The first investigation of this mummy was done in 2002, and later biomedical workups were performed on the same mummy in 2005 [6]. Until the present, at least 9 articles reported the detection of A. lumbricoides eggs either in the coprolites within the mummy’s intestines or in the soil sediment located near the hip bones or sacrum of the dead body [6,18,21,23,26,29,31,33,35]. The surface ultrastructure [20] and molecular characteristics [10,15,16] of A. lumbricoides eggs were also studied. Shin et al. in 2009 [18] reported the recovery of A. lumbricoides eggs in the feces of a mummy from Gongju (Fig. 1, no. 6) dating to the 15th–18th century. Seo et al. in 2010 [21] detected A. lumbricoides eggs in the soil within LSMB tombs having partially preserved mummies at Yongin (HY HM) (Fig. 1, no. 4), Seocheon (Fig. 1, no. 7), and Seoul (Sinnae-dong A, B) (Fig. 1, nos. 8, 9) dating to the 15th–17th century. Shin et al. in 2011 [23] found A. lumbricoides eggs in the mummy soil at Dangjin (Fig. 1, no. 11) dated to 1630s CE. Seo et al. in 2014 [26] and Seo et al. in 2017 [29] added 7 more areas, Paju (Fig. 1, no. 13), Jinju (Fig. 1, no. 14), Yeounggwang-1 (Fig. 1, no. 19), Yeounggwang-2 (Fig. 1, no. 20), Dalsung (Fig. 1, no. 21), Hwasung (Fig. 1, no. 22), and Seoul (Junggye) (Fig. 1, no. 23), where mummies excavated revealed A. lumbricoides eggs dating to the 15th–18th century. The oldest mummy or tomb soil sample examined in Korea (A. lumbricoides egg-positive) was the one obtained from a wooden chamber tomb containing human skeletons in Gyeongju-2 (Fig. 1, no. 25) dating to the Three Kingdom period Silla Dynasty, about 1,500 years before the present (BP) [31]. Studies conducted in 2021 and 2023 added 16th–17th century mummies from Gwangmyeong (Fig. 1, no. 26), Wonju (Fig. 1, no. 28), and Gumi (Fig. 1, no. 29) positive for A. lumbricoides eggs [33,35].
Molecular studiesThe first molecular study on the eggs of Ascaris sp. was performed by Oh et al. [10] who used soil sediment specimens spread on the surface of the hip bones of a mummy in a medieval tomb at Seocheon dating to the 17th century. Ascaris sp. aDNA was successfully extracted in this mummy, and the 18S rRNA and mitochondrial cytochrome b (cyt b) genes were sequenced [10]. Both the 18S rRNA and cyt b gene sequences were 100% identical to those of the Namur samples (Belgium; human origin coprolites) [37], of which the 18S rRNA sequence showed perfect identity with A. suum (GenBank no.: U94367). However, in the cyt b sequence, there were 3 substitutions compared to an isolate of A. suum (GenBank no.: X54253) and 2 substitutions compared to A. lumbricoides [37]. Thus, it was difficult to determine to which species the Namur and Joseon samples belonged [10]. If the synonymy between A. lumbricoides and A. suum [17] is accepted, the Joseon sample [10] could be assigned as A. lumbricoides, according to the rule of taxonomic priority (A. lumbricoides Linnaeus, 1758 vs. A. suum Goeze, 1782). Another molecular study was performed by Oh et al. [15] using the tomb soil from the basal plate of the coffin and the precipitates on the sacrum of a mummy from Seoul dating to the 16th–17th century. Numerous eggs of Ascaris sp. were found microscopically, and their 18S rRNA and cyt b genes were sequenced. According to the results, the 18S rRNA appeared to be not a good genetic locus for differentiation of the Ascaridae species, because this locus could not discriminate even between the genera Ascaris and Baylisascaris [15]. Meanwhile, the sequence of cyt b was useful to differentiate the species of Ascaris, namely, A. lumbricoides (99% identity) and A. suum (97% identity) [15], although these 2 species were suggested to be the same species by other workers [17]. Hong et al. [16] also performed a molecular study on ancient Ascaris eggs targeting several different genetic loci, including cyt b, mitochondrial cytochrome c oxidase subunit 1 (cox1), NADH dehydrogenase subunit 1 (nd1), and internal transcribed spacer 1 (ITS1). They used Ascaris eggs in the feces and precipitates of 8 mummy specimens of the Joseon Dynasty (15th–18th century). The phylogenetic trees of the Ascaris cyt b, cox1, nd1, and ITS1 genes revealed strong clusters of the Joseon samples with A. lumbricoides and A. suum but far from the other Ascaridae species [16].
World reports and evolutionary aspects of Ascaris spp.The evolutionary origin of A. lumbricoides is not precisely understood [38]. The occurrence of this parasite might be related to the establishment of the human agrarian society near the beginning of the Neolithic Era (ca. 10,000 years BP) in Africa. However, the oldest roundworm (Ascaris sp.) eggs ever recorded were in organic remains (sediment and coprolite) of an archaeological site in Grande Grotte, Arcy-sur-Cure, Yonne, France dating to about 30,000 years BP; however, it was impossible to assert whether these eggs were of human or animal (pig) origin [38]. Another discovery of an old Ascaris sp. egg was in the coprolite of a late stone-age man (probably ancestors of modern-day San) recovered from Kruger Cave in the Megaliesberg mountain range, Rustenburg, South Africa dating to 7,000–10,000 years BP [39]. Thus, the historical origin of A. lumbricoides or Ascaris sp. may be at least 10,000–30,000 years BP, before the domestication of pigs, estimated to have begun at around 7,000 years BP [38]. It is generally accepted that this nematode originated in Africa, and it has been found also in archaeological sites of North America and South America, dated as old as 9,000 years ago [38]. Further paleoparasitological studies on archaeological remains >10,000 years will help better understand the evolutionary and geographical origins of human Ascaris. There is a hypothesis that A. lumbricoides originated from A. suum (or vice versa?), which may have occurred some 10,000 years ago [38]. However, these two species were proposed recently to be identical based on molecular studies [17].
Trichuris trichiura and Trichuris sp.General viewsThe human whipworm, T. trichiura (Nematoda: Trichuridae), is another representative species of soil-transmitted helminths [13]. Its geographical distribution, life cycle, biology, and epidemiology are similar to those of A. lumbricoides. The eggs are excreted in the human feces and develop into embryonated eggs in soil [13]. The great majority of T. trichiura infection is moderate or light, with few or no clinical symptoms [13]. However, in heavy infections, diffuse colitis with abdominal cramps and severe rectal tenesmus, chronic dysentery, weight loss, and even rectal prolapse may occur [13]. Trichuris vulpis, the dog whipworm, is a closely related species, and a few human infection cases have been reported [13].
Paleoparasitological studies on Trichuris spp. in KoreaIn Korea, the first detection of ancient T. trichiura eggs was reported by Kwangju National Museum in 1997 from the wetland soil of archaeological remains in Shinchang-dong, Gwangju dating to the Early Iron Age (100 BCE) (Table 2) [4]. In 2003, T. trichiura eggs (49.0±2.7 μm long and 24.8±0.8 μm wide) were discovered again in archaeological soil samples in Chilgok area, Daegu, dating to the Unified Silla Dynasty (668–935 CE) [5]. Since then, at least 25 studies (including the above 2) have been published on the detection of T. trichiura and/or Trichuris sp. (including T. vulpis) eggs (Table 2) [4–6,8,11,14,18–22,24–35,40,41].
When the eggs of T. trichiura are discovered in archaeological samples, they should be differentiated from the eggs of other Trichuris spp. infecting domestic and wild animals, including Trichuris suis (infecting the pig, wild pig, and wild boar), Trichuris ovis (goat, sheep, and cattle), Trichuris globulosa (goat, sheep, cattle, and camel), Trichuris vulpis (dog and fox), Trichuris muris (mouse and rat), and Trichuris arvicolae (field rat) [42,43]. The eggs of T. vulpis (70–89 μm long and 37–40 μm wide), T. ovis (70–80 μm long and 30–42 μm wide), T. globulosa (68 μm long and 36 μm wide), and T. arvicolae (67–79 μm long and 31–43 μm wide) can be differentiated from T. trichiura eggs (45–55 μm long and 25–30 μm wide) by their remarkably larger sizes [22,42,43]. In comparison, T. muris eggs (62–68 μm long and 28–32 μm wide) are only slightly larger than T. trichiura eggs [42,43]. Meanwhile, T. suis eggs (50–60 μm long and 21–25 μm wide) are almost the equal size as T. trichiura eggs, and these two species are morphologically indistinguishable from each other [22,42]. If Trichuris sp. eggs were found in soil specimens from a pit or soil strata containing human excreta, they could be regarded as the human whipworm, T. trichiura [5,19,25]. However, if the archaeological site examined was suggested to be a pigsty, the eggs could be regarded as T. suis eggs. Very large eggs over 70 μm in length with similar morphology could be assigned as T. vulpis or T. ovis eggs. However, it should be noted that there are 2 different groups of T. trichiura eggs, i.e., standard- and large-sized groups, with the large-sized eggs being up to 65–88 μm in length [44]. In addition, T. trichiura eggs may become enlarged in aged female worms, and after treatment with anthelmintic drugs, such as mebendazole or albendazole [45,46]. Therefore, the egg size alone is not a good criterion for determining Trichuris spp., and molecular studies are recommended [11,41].
Soil studiesThe recovery of T. trichiura eggs in ancient soil specimens in Korea has been reported in at least 13 articles [4,5,14,19,22,24,25–28,30,32,34]. Following the first two studies, Shin et al. in 2009 [19] detected a great number of T. trichiura eggs in soil samples from the ruins of a moat encircling the royal palace (Weolseong) of the Silla Dynasty dating to the 5th–8th century. Subsequently, T. trichiura eggs (Fig. 4A) were found in strata soil or streambed soil from Seoul dating to the Joseon Dynasty (14th–19th century) [22,24] and in archaeological site soil from Jangheung (Fig. 1, no. 12) dating to the 19th century [25]. Further, T. trichiura eggs were detected in soil samples from V-shaped pits (presumed to be human toilets) [14] and geological strata [27] dating to the Baekje Dynasty (6th–7th century). In addition, T. trichiura eggs were found also in soil sediment samples from Gimhae and Changnyeong dating to ~ 676 CE of the ancient Silla Dynasty [28], Seoul dating to the Joseon Dynasty (15th century) [30], and Buyeo-2 and Buyeo-3 dating to the 5th–7th century of the Baekje period [32,34].
Interestingly, Trichuris sp. eggs, as large as 72–89 μm long and 37–40 μm wide, were detected in soil samples from yards, ditches, and toilets of Seoul dating to the 15th century and were assigned to T. vulpis eggs [30]. Similar large-sized Trichuris sp. eggs were also found in soil specimens from possible toilets in Buyeo-2 [32] and Buyeo-3 (Hwajisan) (Fig. 1, no. 27) [34] (Baekje Dynasty) dating to the 6th–7th century.
Mummy studiesFrom mummy specimens, the eggs of T. trichiura were first detected in 2007 in fecal samples of a child mummy from Yangju dating to the 17th century [6]. Including this report, at least 10 articles have been reported regarding the detection of T. trichiura eggs either in the feces or coprolites within the mummy’s intestines or in the mummy soil near the hip bones, sacrum, or basal layer of the dead body [6,8,18,21,26,29,31,33,35,40]. Lee et al. in 2009 [8] detected T. trichiura eggs in the feces of a male mummy excavated at Gangneung (Fig. 1, no. 30) dating to 1622 CE. In the same year, Shin et al. [19] detected T. trichiura eggs in the mummy soil from Gongju dating to the 17th–18th century. Seo et al. in 2010 [21] detected T. trichiura eggs in the soil within LSMB tombs, where partially mummied bodies were found, from Yongin, Seocheon, Waegwan (Fig. 1, no. 31), and Seoul (Sinnae-dong A-D, Fig. 1, nos. 8, 9, 32, and 33) dating to the 15th–17th century. Further, Shin et al. [40] detected T. trichiura eggs (Fig. 4B) in the feces of a male mummy from Sapgyo (Fig. 1, no. 34) dating to the 16th century. After that, Seo et al. [26] and Seo et al. [29] added 9 more mummies from Jinju, Mungyeong (Fig. 1, no. 35), Paju, Andong (Fig. 1, no. 36), Yeounggwang-1, Yeounggwang-2, Dalsung, Hwasung, and Seoul (Junggye) dating to the 15th–18th century. The organic material (precipitates) on the hip bones and sacrum in a dead human body excavated at Gyeongju-2 dating to the 6th century (Three Kingdom period, Silla Dynasty) was the oldest human-source archaeological material ever examined in Korea in which T. trichiura eggs were found [31]. Oh et al. [33,35] added 6 more mummies positive for T. trichiura eggs from Goryeong (Fig. 1, no. 37), Gwangmyeong, Wonju, Euijeongbu-1 (Fig. 1, no. 38), Euijeongbu-2 (Fig. 1, no. 39) and Gumi. The surface ultrastructure of T. trichiura eggs was studied by Shin et al. in 2009 [20] using the eggs obtained from the feces of the Yangju mummy [6].
Molecular studiesMolecular studies on Trichuris spp. eggs were reported twice in Korean literature [11,41]. The first study was conducted by Oh et al. [11] who used the mummy soil spread on the surface of the sacrum and several places on the basal plate of a female mummy in a medieval tomb from Seoul (Sinnae-dong) dating to the 18th century. Trichuris sp. aDNA was extracted, and the small subunit rRNA (SSU rRNA) gene was successfully amplified and sequenced [11]. Results revealed that the sequence of the SSU rRNA gene was 100% identical to that of T. trichiura in GenBank (no.: DO118536.1), whereas 97% and 91% homologies were found between the tested sample and T. suis and between the tested sample and T. muris, respectively [11]. Another study on the molecular diagnosis of T. trichiura eggs was performed by Hong et al. [41] who used the fecal samples or sediments precipitated on the hip bones of 8 mummies in Korea (Jinju, Junggye, Waegwan, Sinnae-dong A, Mungyeong, Seocheon, Yeounggwang-1, and Yeounggwang-2) dating to the 15th–18th century. The genetic loci examined were SSU rRNA, ITS2, and ATP synthase subunit 8 (ATP8) and were proved to be highly useful in differentiating T. trichiura from other Trichuris spp. [41]. The sequences of SSU rRNA and ITS2 genes in the Joseon specimens were closely clustered with those of T. trichiura in GenBank (Japan, China, Thailand, England, Uganda, Ancient Korea, and Ancient Denmark) but distinct from the sequences of other Trichuris spp., including T. suis and T. muris [41]. The sequence of ATP8 gene was highly homologous with a group of T. trichiura in GenBank (China and Japan) but a little distant from another group of T. trichiura in GenBank (Uganda, Ancient Denmark, and Ancient Netherlands) [41]. These results supported the suggestion that there are two different genetic clades in T. trichiura as revealed by ITS1 gene sequences; one ubiquitous and the other restricted to medieval Lübeck (Germany) and Bristol (England) [47].
World reports and evolutionary aspects of Trichuris spp.The historical origin of T. trichiura is not precisely understood [38]. However, the occurrence of this parasite has been thought to be the establishment of human agrarian society in the eraly Neolithic Era (ca. 10,000 years BP). The oldest T. trichiura eggs ever documented globally were 3 eggs found in the coprolite of a late stone-age man, recovered from Kruger Cave, Rustenburg, South Africa dating to 7,000–10,000 BP [38,39]. The next oldest T. trichiura eggs were recorded from the probable human coprolites in latrines and cesspits in Lapa Pequena, Minas Gerais, Brazil dating to 7,000–8,000 BP [39]. This nematode species has been evidenced to exist in North America and South America as old as 9,000 years ago [38]. According to a previous concept, the human whipworm species was thought to have been derived from T. suis [48]. Later, however, the evolutionary origin of T. trichiura was suggested to be much older (at least 30,000 years), preceding the domestication of pigs, and this nematode species originated from African human ancestors [38,48].
Strongyloides stercoralis and Trichostrongylus sp.General viewsThere are two life cycle generations in S. stercoralis (Nematoda: Strongyloididae). One is the free-living generation in soil, and the other is the parasitic generation in the intestines of humans, dogs, and cats [13]. In the free-living generation, males, females, and larval forms can be found, whereas, in the parasitic generation, only females (reproduce parthenogenetically) and larvae are found [13]. Therefore, in paleoparasitological studies, caution should be paid to the differential diagnosis of parasitic S. stercoralis adults (females) and larvae with those of the free-living generation. In addition, they should be further differentiated from other Strongyloides spp., and species of Rhabditis, hookworms, and Trichostrongylus. In immunosuppressed or immunodeficient human patients, S. stercoralis may cause fulminant infections attacking various organs and tissues leading to the hyperinfection syndrome, which is often life-threatening [13].
Trichostrongylus spp. (Nematoda: Trichostrongylidae) are intestinal nematodes infecting humans (T. orientalis, T. colubriformis, and some other species) and animals (numerous species are known). Infection may occur by ingestion of their eggs in soil or contaminated vegetables [13]. Human infections with these nematodes are in most cases asymptomatic unless they are very heavily infected.
Paleoparasitological studies on Strongyloides stercoralis and Trichostrongylus sp. in KoreaIn Korea, the larvae of S. stercoralis and the larvae of Trichostrongylus sp. were found together one time in an archaeological specimen (Table 3) [18]. The sample was the soil spread upon the hip bones and sacrum of a male mummy from Gongju dating to the 17th–18th century. S. stercoralis larvae were 365 μm long and 20 μm wide having a rhabditiform esophagus and other characteristics, and these were assigned to the 1st stage rhabditoid larvae of S. stercoralis [18]. Trichostrongylus sp. larvae were 330–373 μm long and 18–23 μm wide and had a rhabditiform esophagus [18]. These 2 species of larval nematodes could be differentiated by a much greater length of the buccal cavity in Trichostrongylus sp. than in S. stercoralis. In addition, unlike S. stercoralis larvae, a bead-like swelling was observed in the caudal tip of the long, slender tail of Trichostrongylus sp. larvae [18].
World reports and evolutionary aspects of Strongyloides stercoralis and Trichostrongylus sp.The oldest S. stercoralis larvae (Strongyloides sp.?) ever discovered in the world were those from an Egyptian mummy known as Asru dating to about 1,000 BCE [39]. Four more reports have been published on the detection of S. stercoralis larvae in the USA and Netherlands; however, the specific diagnosis was uncertain in some of them [39,49]. One report was on the finding of the 1st stage larvae of Strongyloides sp. (260–360 μm long) in a human coprolite and two dog coprolites obtained from Antelope House in Canyon de Chelly National Monument, Arizona, USA dating to 1175–1250 CE; there was no evidence of free-living nematode penetration into the cave environment [49].
The report of the oldest Trichostrongylus sp. egg was from archaeological remains (100 feces samples) in Dust Devil Cave, Utah, USA dating to 6,800–8,800 years BP; however, the egg detected was only 1 in number, and the diagnosis was tentative as it was thought probably not a human parasite [39,50]. Four more reports have been available on the recovery of Trichostrongylus sp. eggs in the USA, Mexico, Argentina, Chile, and Brazil but the diagnosis was uncertain on two occasions [39,50]. One report was in coprolites from Antelope House in Arizona, USA dating to 1175–1250 CE in which 44 eggs (78×45 μm) of Trichostrongylus sp. (tentative diagnosis) were collected from 1 of the 62 fecal samples of human or dog origin [49].
HookwormsGeneral viewsHookworms are a group of soil-transmitted helminths. The species infecting humans in their adult stage are mainly Ancylostoma duodenale and Necator americanus, and rarely Ancylostoma ceylanicum and Ancylostoma caninum (Nematoda: Ancylostomatidae) [13]. They parasitize the small intestine and suck blood which leads to anemia, hypoxia, and growth retardation in infected patients, particularly, children [13].
World reports and evolutionary aspects of hookwormsThe eggs of hookworms have been found in archaeological remains most commonly in North and South Americas, rarely in Europe and Africa, but never in Asia, including Korea [39]. The presence of hookworm eggs in prehistoric populations in northeast Brazil dating to 7,230±80 years BP suggests that the evolutionary origin of hookworms would have been long [39,51], possibly 10,000–30,000 years BP. The geographical origin of A. duodenale was suggested to be at some points in northern Africa, southern Europe, or Asia north of Himalayas from Homo sapiens (not confirmed) [48,51]. The origin of N. americanus was suggested to be the southern region of the Sahara Desert in Africa probably from a pre-hominid ancestor, i.e., African ungulates [48]. It seems unlikely that hookworms could survive the severe climate of northern North America when ancient people migrated through the Bering Strait; thus, it was suggested that the hookworms came to the New World by way of the sea from countries in Asia or Indochina, e.g., by ‘storm-tossed fishermen’ [51].
No discovery of ancient hookworm eggs in KoreaIt is unclear why hookworm eggs (or larvae) have not been detected in archaeological remains in this country. However, it can be considered that hookworm eggs have a very thin and weak eggshell (chitin layer), and they may be more fragile than A. lumbricoides and T. trichiura eggs which have a thick and strong chitinous wall. Moreover, hookworm eggs develop quickly in the feces to form the 1st stage larvae inside the eggs, and these larvae readily break the eggshell and go out into the environment. The eggs and larvae are easily destroyed in the feces unless dispersed into the moist, sandy soil where they can survive and develop further if the temperature and humidity are appropriate. However, genetic materials may remain in the feces or coprolites of archaeological remains, and molecular techniques to detect the aDNAs of hookworms may be helpful in Korea and other countries.
Paracapillaria philippinensis and other capillariid nematodesGeneral viewsAmong the capillariid nematodes (Nematoda: Capillariidae), species infecting humans include Calodium hepaticum (syn. Capillaria hepatica) that parasitizes the liver parenchyma, Paracapillaria philippinensis (syn. Capillaria philippinensis) that infects the small intestine, and Eucoleus aerophilus (syn. Capillaria aerophila) that infects the respiratory tract [13,52–55]. C. hepaticum is a parasite of rodents and other carnivorous mammals; although rare, human infections (mostly children) were reported in Korea, India, the USA, Türkiye, South Africa, Mexico, Brazil, Italy, the Czech Republic, Nigeria, Switzerland, and Japan [54]. The patients develop acute and subacute hepatitis with hepatomegaly and liver dysfunction [13]. P. philippinensis is a parasite of exclusively humans causing fatal diarrhea reported originally in the Philippines in the 1960s [13]. This parasite may cause symptoms like abdominal discomfort, chronic diarrhea, borborygmus, and severe intestinal malabsorption; death may occur due to complications like pneumonia, heart failure, hypokalemia, or cerebral edema [13]. It has been subsequently found in Thailand, Iran, Egypt, Taiwan, Japan, and Korea, possibly transported by migrating birds [55]. In human capillariid infections, it should be remembered that spurious (false) infection may occur by accidental ingestion of infected fish or mammals, the definitive host for various species of Calodium and Paracapillaria [42].
Paleoparasitological studies on Paracapillaria philippinensis in KoreaIn Korea, the eggs of Paracapillaria sp., possibly P. philippinensis [52], were found once in an archaeological specimen (Table 3) [18]. The sample was the soil spread upon the hip bones and sacrum of a male mummy from Gongju dating to the 17th–18th century. The eggs (34–35 μm long and 17–20 μm wide) had relatively broad mucoid plugs at both ends; however, striations or ornamentation networks, or small mamillated eggshells (characteristic structures) were not easily recognized [18]. They looked like P. philippinensis, C. hepaticum [52], or other capillariid species eggs. However, C. hepaticum eggs are larger (48–65 μm long and 28–35 μm wide) and almost elliptical [54], whereas P. philippinensis eggs (41–48 μm long and 19–20 μm wide) are octagon-like with less prominent striations or ornamentations on the shell [55]. Thus, the eggs detected in Korea were likely those of P. philippinensis. Additionally, the specimens in Korea were collected from the lower abdominal area, but not from the hepatic region. Therefore, the possibility for C. hepaticum could be ruled out [19]. Contemporarily in Korea, a few human infection cases with P. philippinensis were reported [55,56].
World reports and evolutionary aspects of capillarid nematodesThe finding of capillariid nematode eggs in archaeological remains of human origin has been relatively rare worldwide [53,57–59]. Approximately, 11 articles have been published on the recovery of C. hepaticum or Calodium sp. eggs from human (5 reports) and animal remains (6 reports) [53]. An old report in 1968 mentioned the recovery of capillariid (or trichuriid) eggs in the pit soil from Winchester, England dating to the Roman age (700 BCE-476 CE); the eggs were 50–58 μm long and 23 μm wide [57]. The eggs might have been those of C. hepaticum or Calodium sp. based on their size; however, the ultimate diagnosis was not drawn [57]. Another study worth mentioning is an article reported in 1997 in which two types of capillariid eggs (62.0×35.0 μm and 70.0×31.5 μm in size) were discovered in 21 of 23 human coprolites taken from the neolithic Chalain Lake settlement, Jura, France dating to 3200-2980 BCE [58]. We regarded these eggs as those from intestinal capillariasis (i.e., P. philippinensis infection), although the species name was not precisely given [58]. In 2020, capillariid eggs (60.9±1.2 μm×32.8±1.9 μm) were discovered in the organic abdominal content of a mummy from Bolivia dating to 1150–1450 CE; since these are wider than P. philippinensis eggs, they may be C. hepaticum or Calodium sp. [59].
Enterobius vermicularisGeneral views
Enterobius vermicularis (Nematoda: Oxyuridae), the human-infecting pinworm, is a cosmopolitan species and is prevalent among families, schools, and asylums, and the infection is more common in children than in adults [13]. This nematode is transmitted by a contaminated finger (anus-to-mouth transmission), body contact with other persons, or through vehicles, such as bed linens, tabletops, doorknobs, and other contaminated objects [13]. It infects the large intestine and can cause perianal itching and dermatitis [13].
Paleoparasitological studies on Enterobius vermicularis in KoreaThe eggs of E. vermicularis were found once in coprolites of a female mummy from Dangjin dating to the 1630s CE (Table 3) [23]. The coprolites were obtained from the luminal surface of the ascending, transverse, and descending colons. Three E. vermicularis eggs (50.3 ±5.2 μm long and 28.2±3.9 μm wide) were found [23]. This was the second discovery of pinworm eggs in archaeological remains in East Asia, following the report from a 2100-year- old female mummy in Hunan province, China [60].
World reports and evolutionary aspects of Enterobius vermicularisThe recovery of E. vermicularis in archaeological remains has been reported most frequently in North and South Americas followed by a few reports in Europe, Asia (China and Korea), Africa (Egypt), and the Middle East [23,39,60–62]. The oldest E. vermicularis eggs ever recorded were those found in North American indigenous groups from the Great Basin Region [39,63]. Human coprolites from Danger and Hogup Caves, western Utah, USA dating to 7,837 BCE showed E. vermicularis eggs; the caves were inhabited by humans from 10,000 BCE to 1,400 CE [63]. Pinworms seem to be the oldest nematode parasitic in humans (several million years old), and the evolutionary origin of E. vermicularis is suggested to predate humans [48]. They evolved previously with pre-hominid hosts and thereafter mutually with humans [48]. This nematode originally came from Africa and is thought to have spread to Asia, Europe, and North and South America [48,64,65]. Thus, E. vermicularis, as well as T. trichiura, Dibothriocephalus spp. (syn. Diphyllobothrium spp.), and Trichinella spiralis, were regarded as examples of heirloom parasites originating from human antecedents [64]. The introduction of E. vermicularis into the Americas was suggested to have been both by prehistoric human migrations across the Bering route and transpacific contacts of Asian people with the Aboriginal Americans [48,65]. In vertebrate hosts other than humans, the origin of pinworms was suggested to be much older, up to 240 million years, since an egg of an ancient pinworm, Paleoxyuris cockburni n. sp. (order Oxyurida Railliet, 1916) was discovered in the coprolite of a primitive protomammal (a species of dinosaurs) of Class Cynodontia [66].
Fasciola hepatica and F. giganticaGeneral views
Fasciola hepatica and F. gigantica (Digenea: Fasciolidae) are the largest in size of all trematode species infecting humans and animals [13]. Their normal habitat is the liver (bile duct) [13]. Transmission of these flukes occurs through the consumption of aquatic vegetables, including watercress, water bamboo, water caltrop, hyacinth, morning glory, and so on. Animals and humans infected with these flukes may develop severe liver diseases. In humans, these flukes may also cause ectopic parasitism in the blood vessels, lungs, subcutaneous tissues, brain, and orbits [13]. False parasitism (spurious infection) may occur when the infected animal liver is consumed, with the passage of the eggs in the feces, which is at times mistaken for an actual infection [13].
Paleoparasitological studies on Fasciola hepatica in KoreaIn Korea, F. hepatica eggs were reported once in an archaeological human residence during the 15th century in Seoul (<1 km from the Gyeongbok Palace of the Joseon Dynasty) (Table 4) [30]. Most of the eggs (n=8), with an average size of 140×80 μm, had lost their operculum, and were found in soil samples from a ditch probably connected to the human latrine [30]. A. lumbricoides eggs were found simultaneously in the same ditch; hence, these F. hepatica eggs may have been from a genuine human infection or possibly a spurious infection in humans. However, in human mummies in Korea, F. hepatica eggs have not yet been discovered [9].
World reports and evolutionary aspects of Fasciola spp.The oldest eggs of F. hepatica ever found in archaeological samples worldwide were those in human remains from Cyprus dating to 9,000–10,300 years BP [67]. The next oldest F. hepatica eggs were found in coprolites from Clairvaux, Jura, France, the estimated age being 5,600 years BP [39,68]. F. hepatica eggs of similar archaeological ages were also reported from the Netherlands (5,230–5,400 years BP), Switzerland (5,384–5,370 years BP and 5,176–5,153 years BP), and France (5,200–4,980 years BP) [39,68,69]. Notably, remains of a part of an adult F. hepatica worm were discovered in the liver tissue of an Egyptian mummy dating to 4,600 years BP [70]. The geographical origin of fasciolid trematodes (for example, the basal species Protofasciola robusta infecting African proboscideans, such as elephants) was suggested to be a southern part of Africa (ca. 100 million years BP) [71]. F. gigantica is thought to have derived from the ancestors of F. nyanzae (infecting the hippopotamus) in southeastern Africa, i.e., Uganda, Congo, Kenya, Tanzania, Zambia, and Zimbabwe, by a host capture phenomenon from early hippopotamids to ancient bovids around 13.5 million years BP, the mid-Miocene era [72]. F. hepatica is thought to have been split from F. nyanzae ancestors or F. gigantica in the Near East or some areas of Europe or Asia 5.3–10 million years BP, and then both species were reintroduced into Africa with the migration of bovid hosts [71,72]. F. hepatica-infected bovids also migrated from Asia to North America crossing the Bering land bridge 2–5 million years BP and began to adapt to local lymnaeid snails (~ 2 million years BP); this is thought to be the first migration of F. hepatica to the Americas and is backed up by the fact that F. hepatica aDNA was found in coprolites of native deer in South America dating to 2,300 years BP [71]. A second introduction of F. hepatica into the Americas is thought to have occurred around 1500 CE since the early European colonization of the Americas [71].
Dicrocoeliid trematodesGeneral viewsThe Dicrocoeliidae family (Digenea) is comprised of at least 9 species of medical and veterinary importance, including Dicrocoelium dendriticum, D. hospes, Platynosomum fastosum, and Eurytrema pancreaticum [42,73]. The most important species worldwide is the lancet liver fluke D. dendriticum (egg size, 39–46×26–30 μm) [74]. However, in Korea, D. dendriticum has never been found, and the pancreatic fluke E. pancreaticum (egg size, 43–69×28–35 μm) is commonly observed [74]. D. dendriticum and E. pancreaticum infect the bile duct and pancreatic duct of domestic animals, including cattle, goats, sheep, and deer, and cause liver and pancreatic diseases, respectively [13]. However, these flukes can also infect humans if they accidentally eat the infected ant (D. dendriticum) or other kinds of insects (grasshopper or cricket) (E. pancreaticum) [73].
Paleoparasitological studies on dicrocoeliid nematodes in KoreaIn Korea, dicrocoeliid trematode eggs were found once in soil sediment samples from Gimhae, an archaeological site of the ancient Silla Kingdom dating to 57 BCE–975 CE [28] (Table 4). It was difficult to determine the exact period of the soil sediment samples. The average egg size was 41.7×26.0 μm [28], which was more compatible with D. dendriticum than E. pancreaticum eggs [13,28,74]. However, it needs to be verified whether D. dendriticum existed in Korea in ancient times. As the examined specimen was the soil sediments formed by flood precipitation, it was uncertain whether the origin of these eggs was humans or domestic animals. Even if these eggs were of human origin, a pseudo-parasitosis (spurious infection) should be ruled out [73].
World reports and evolutionary aspects of dicrocoeliid nematodesThe lancet liver fluke is widely distributed in Europe and Asia and sparsely in Africa, North America, South America, and Australia [73]. The oldest evidence of this trematode infection in the world is the discovery of dicrocoeliid eggs in the coprolites and their underneath soil sediment at the site of the Caune de l’Arago, a Middle Pleistocene cave near Tautavel, France [73,75]. However, the samples were considered probably of animal origin, dating back to ~550,000 years ago [73,75]. The next oldest dicrocoeliid eggs recorded were those from Arbon, Thurgau, Switzerland, and Chalain, Jura, France dating to 3384-3370 BCE and 3040-3000 BCE, respectively [39]. Dicrocoeliid eggs were also discovered in human excrements at the Hallein salt mines, near Salzburg, Austria dating to 500–200 BCE [39,73]. Subsequently, England and Denmark were added among the countries where dicrocoeliid eggs were discovered in archaeological remains [76]. All these reports were from Europe, and only a few were from Asia, Africa, and the Americas. Several studies were performed in Russia and Iran [76–78]. Interestingly, a recent report from Taiwan described the discovery of Eurytrema sp. eggs (possibly E. pancreaticum) in the sediment from a colonial-period latrine in Batongguan Trail, Central Mountain Range, mid-eastern Taiwan [79]. The eggs were 44–50×27–33 μm in size, larger than those of D. dendriticum [79], and were found in cesspool sediments and probably of human origin.
Clonorchis sinensis and Opisthorchis spp.General views
Clonorchis sinensis, Opisthorchis viverrini, and O. felineus (Digenea: Opisthorchidae) are small liver flukes infecting humans and animals. Their life cycles are completed through passages in 3 sequential hosts, including freshwater snails (first intermediate host), freshwater fish (second intermediate host), and fish-eating mammals, including humans (definitive host) [13]. Human infection occurs through the ingestion of raw or incompletely cooked freshwater fish, and the parasites live in the liver-biliary system [13]. The parasites induce inflammation in the bile duct (cholangitis), and in chronic infections, they lead to liver cirrhosis and even cholangiocarcinoma which is often fatal [13,74]. C. sinensis is prevalent in the Far East (Korea, China, and Russia) and East Asia (Vietnam), O. viverrini occurs in the Great Mekong Subregion and Indochina peninsula (Thailand, Lao PDR, Vietnam, Cambodia, Malaysia, and Myanmar), and O. felineus is distributed in Eastern and Southern Europe and Central Asia (Russia, Germany, Greece, Poland, Romania, Italy, Spain, Belarus, Ukraine, and Kazakhstan) [80].
Paleoparasitological studies on Clonorchis sinensis in KoreaIn Korea, the first detection of ancient C. sinensis eggs (27.7±2.3 μm long and 18.8±4.8 μm wide) was reported by Han et al. [5] in 2003 in archaeological soil samples from Chilgok area, Daegu, dating to the Unified Silla Dynasty (668–935 CE). Since then, at least 16 documents, including Han et al. [5], have been published (Table 4) [5–7,12,14,21,22,26,29, 30,32,34,35,40,81,82].
When the eggs of C. sinensis are found in archaeological samples, they should be differentiated from those of other opisthorchiid flukes (O. felineus and O. viverrini) and heterophyid (Metagonimus spp., Heterophyes spp., and others) and lecithodendriid flukes (Caprimolgorchis molenkampi and Phaneropsolus bonnei) [81,82]. C. sinensis eggs could be differentiated from the others by size, shape, and other morphologies with some difficulty. One of the most distinguished features is the presence of prominent shoulder rims and strong muskmelon patterns (surface elevations on the eggshell) in C. sinensis eggs [20,84]. In comparison, O. viverrini and O. felineus eggs have less prominent shoulder rims and less marked muskmelon patterns. In addition, heterophyid and lecithodendriid fluke eggs lack shoulder rims and have relatively clean eggshell surfaces [83,84]. However, in old archaeological samples, the eggshell surface elevations in C. sinensis eggs tend to change a little to become less marked [7]. In such situations, the geographical locality where the eggs were found can help the differential diagnosis with other opisthorchiids, since the endemic areas of C. sinensis, O. felineus, and O. viverrini are unique with no overlaps except in Vietnam where both C. sinensis (in northern parts) and O. viverrini (in southern parts) are distributed [80,85].
Soil studiesIn Korea, the recovery of C. sinensis eggs in ancient soil specimens has been reported in at least 6 articles [5,14,22,30,32,34]. Following the first report [5], Shin et al. in 2011 [22] detected a small number of C. sinensis eggs in soil samples from archaeological sites (road near the Palace of the Joseon Dynasty) at Seoul dating to the 16th–18th century. After this, C. sinensis eggs (Fig. 5A) were repeatedly found in soil samples obtained from geological soil strata [22], V-shaped pits (presumed to be toilets) [14], a toilet and its drainage duct excavated at Buyeo-2 [32], and soil samples at Buyeo-3 (Hwajisan), Baekje Kingdom [34]. In addition, C. sinensis eggs, 6 in number, were detected in soil samples from an outlet of a toilet (?) in Seoul dating to the 15th century [30].
Mummy studiesIn mummy samples, C. sinensis eggs were first found in a specimen named ‘Hakbong mummy’ by a Korea University Research Team in 2004 [7]. Unfortunately, however, this finding has not been officially published. The first official publication of the recovery of C. sinensis eggs in a mummy specimen was in 2007 in a child mummy from Yangju dating to the 17th century [6]. It is suggested that dishes of raw or undercooked freshwater fish may have been popularly available even to the children of the medieval period in Korea. Since then, at least 10 mummy studies, including the first one, were reported on the detection of C. sinensis eggs either in the feces/coprolites of the mummy’s intestines or in the soil sediment near the hip bones, sacrum, or basal layer of the mummy [6,7,12,21,26,29,35,40, 81,82]. Importantly, Seo et al. [7] found C. sinensis eggs (28.8±1.3×15.9±0.7 μm) in the feces of a 16th- to early 17th-century female mummy at Hadong-1 (Fig. 1, no. 40), a coastal county where the Seomjin River flows into the South Sea, Korea. This mummy was perfectly protected from the outside by an LSMB layer, and the feces were sampled from the luminal surface of the rectum. Hadong County is even at present recognized as an endemic area of C. sinensis and M. yokogawai infections [7]. Subsequently, Seo et al. in 2010 [21] detected C. sinensis eggs (Fig. 5B) in the soil within an LSMB tomb containing a partially preserved mummy at Waegwan dating to 1685 CE. Waegwan Town is located near the upper reaches of the Nakdong River where C. sinensis infection is still prevalent. In addition, Shin et al. [40] detected C. sinensis eggs in the feces of a male mummy at Sapgyo dating to the 16th century in 2012. Seo et al. [26] added Mungyeong among the areas where C. sinensis eggs were detected in a mummy dated to 1647 CE in 2014. Mungyeong City is located near the upper reaches of the Nakdong River. In 2017, Seo et al. [29] added another area, Andong, where C. sinensis eggs were detected in a mummy’s fecal samples dating to the 16th century. Andong City is also located near the upper reaches of the Nakdong River. Hong et al. [81] added 2 mummies from Cheongdo (Fig. 1, no. 24) (17th century) and Dalsung (16th–17th century) in 2019 from which C. sinensis eggs were detected and molecularly analyzed. These 2 areas are near the tributaries of the Nakdong River. Oh et al. in 2022 [82] and 2023 [35] added 3 more mummies from Goryeong, Wonju, and Gumi, in which C. sinensis eggs were detected, dating to the 16th–17th century. Goryeong County is near the middle reaches of the Nakdong River. Wonju City is about 25 km away from the Namhan River (=South Han River), and Gumi City is near Waegwan Town at the upper reaches of the Nakdong River, all of which are currently low to moderate endemic areas of C. sinensis infection.
Molecular studiesShin et al. [12] first analyzed the sequences of ITS1, ITS2, and cox1 genes of C. sinensis eggs obtained from the Waegwan mummy [21]. The results revealed 100% homology to contemporary C. sinensis gene sequences [12]. Hong et al. [81] also analyzed the sequences of ITS1, cox1, and NADH dehydrogenase subunits 2 and 5 (nd2 and nd5) genes of C. sinensis eggs acquired from the coprolites of Cheongdo [81], Dalsung [81], Andong [29], Hadong-1 [7], and Mungyeong [26] mummies. The identities of the gene sequences between the ancient and modern C. sinensis eggs were 96–100% by cox1, 98–100% by ITS1, 98–99% by nd2, and 99–100% by nd5 [81]. Later, Oh et al. [82] also analyzed the ITS1, cox1, nd2, and nd5 sequences of C. sinensis eggs obtained from a mummy in Goryeong. The identities of the gene sequences between the ancient and modern C. sinensis eggs were 98.2–100% by ITS1, 99.4–100% by cox1, 99.4–100% by nd2, and 99.1–100% by nd5 [82], giving similar results with Hong et al. [81].
World reports and evolutionary aspects of small liver flukesThe evolutionary origin of opisthorchiid liver flukes, including C. sinensis and O. felineus, was studied recently [86]. C. sinensis and O. felineus were thought to have split from their last common ancestor infecting carnivorous mammals about 3 million years ago, based on an analysis of mitochondrial DNA sequences [86]. It was further speculated that modern humans who migrated out of Africa and settled in East Asia by at least 60,000 years BP may have been first exposed to C. sinensis, probably in China [86].
However, the oldest eggs of C. sinensis ever recorded from archaeological remains worldwide were those found in a female mummy dating to the Chu Dynasty, China (Warring States period; 475-221 BCE), about 2,500 years BP [87–89]. Thereafter, in China, C. sinensis eggs were found in at least 8 mummies and hygiene sticks in a latrine dating from the Han Dynasty (206 BCE–220 CE) to the Qing Dynasty (1644–1912 CE) [88]. In Japan, C. sinensis eggs were discovered in an early medieval cesspit of the Fujiwara Palace Site at Kashihara City, Nara Prefecture dating to the 7th century [90], but no mummy studies were reported. Interestingly, in the USA, C. sinensis eggs, together with A. lumbricoides and T. trichiura eggs, were found in 1880–1930s’ sediment samples from a latrine used by Chinese Americans on the property of Wong Nim, an important member of the Chinese community in San Bernadino, California [91]. Similar old infections with C. sinensis were also confirmed in Sacramento, California [91].
There are a few paleoparasitological reports on Opisthorchis spp. liver flukes, O. felineus and O. viverrini, which do not exist in Korea. Early archaeological studies reported that the eggs of Opisthorchis sp. (probably O. felineus) or Opisthorchiidae were found in coprolites (mostly of human origin) from Swifterbant Culture Sites, the Netherlands dating to 5400±40 BCE and 5230±40 BCE [39,92] and from archaeological remains in Arbon, Thurgau, Switzerland dating to 3384-3370 BCE [39,93]. A fluke egg, 35×18 μm in size, was also found in the coprolite (probably of human origin) from Glen Canyon, Utah, USA dating to 1250 CE [94]; however, it might have been a spurious infection [95]. In Russia, O. felineus (less probably Metorchis bilis) eggs were recovered from the pelvic and/or abdominal areas of an infant [96] and a child (6–7 years old) body remains [97] buried in Zeleniy Yar archaeological site, Yamalo-Nenetz Autonomous Okrug in the north of western Siberia dating to the 12th–13th century. O. felineus eggs were also recovered in archaeological soil specimens (humans and dogs) from cultural layers of Nadym Gorodok, western Siberia, dating to the 14th-late 18th centuries [98]. In addition, O. felineus eggs were found in mummy specimens of Siberian natives before and after (Khanty and Mansi people) contact with Russians and Russian immigrants-descendants (Russian old-timers or Yeniseisk), implying that O. felineus infection was widely prevalent in the Siberian people [99]. There are almost no paleoparasitological reports on O. viverrini. This species is considered to have split from C. sinensis somewhere in Southeast Asia, possibly in the Indochina peninsula, a long time ago. Notably, after the 1950s, the endemic areas of O. viverrini moved from the northeastern areas of Thailand to the central regions according to the immigration of northeastern Thai people to the central areas for better living [100].
Paragonimus westermani and other Paragonimus spp.General views
Paragonimus spp. (Digenea: Paragonimidae) are lung flukes infecting humans and animals [13]. The most widely distributed species is P. westermani, and numerous high-endemic areas are found in Asian countries [80]. In Asia, 3 more Paragonimus spp. are distributed causing human paragonimiasis, including P. heterotremus (China, Vietnam, Lao PDR, Thailand, Myanmar, India, and Sri Lanka), P. skrjabini (China, Vietnam, and India), and P. skrjabini miyazakii (Japan) [80]. In America, two species, namely, P. kellicotti (the USA and Canada) and P. mexicanus (South America), and in Africa, 3 species, P. africanus (Guinea, Cameroon, Nigeria, and Ivory Coast), P. uterobilateralis (Cameroon, Nigeria, Liberia, Guinea, Ivory Coast, and Gabon), and P. gondwanensis (Cameroon) are known to occur [80]. Their first and second intermediate hosts are freshwater snails and crabs/crayfish, respectively, and their definitive host is carnivorous mammals, including dogs, cats, pigs, leopards, tigers, foxes, wolves, and humans [13,80]. The mode of human infection is the consumption of raw or undercooked freshwater crabs and crayfish or eating undercooked wild boar meat [80]. These flukes cause infection in the lung parenchyma and induce bronchitis and bronchiolitis with symptoms (cough, bloody sputum, bronchiectasis, low-grade fever, and chest pain) like those observed in pulmonary tuberculosis [13,80].
Paleoparasitological studies on Paragonimus westermani in KoreaIn Korea, the ancient P. westermani eggs have been reported from 10 mummy samples and 1 archaeological soil sample from 2009 until 2023 which were published in 9 articles [18,21, 23,26,30,35,101–103] (Table 5). The first detection of P. westermani eggs (80.0–82.5 μm× 50.0–55.0 μm) was reported by Shin et al. in 2009 [18] in the soil spread upon the hip bones of a male mummy at Gongju dating to the late 17th century. The next report was published by Seo et al. in 2010 [21] from the mummy soil on the surface of hip bones and sacrum of a half-mummified body at Yongin dating to the 15th–16th century. The 3rd recovery of P. westermani eggs (Fig. 6A) was done from the luminal surface of ascending, transverse, and descending colons of a female mummy at Dangjin dating to 1633 CE [23]. It is considered that these 3 cases were pulmonary paragonimiasis, and probably the eggs in sputum were swallowed and detected in the mucosal surface of the colon and feces. The next case was a 400-year-old female mummy found at Hadong-2 (Fig. 1, no. 41) who suffered from ectopic paragonimiasis in the liver combined with a pulmonary infection [101]. In this case, P. westermani eggs (Fig. 6B) were numerous and detected in the precipitates of the lungs, feces, intestine, liver, and lung- and liver-tissue sections; molecular studies were done in this case [101]. The 5th case was a half-mummified body found at Jinju dating to the 15th–16th century, and P. westermani eggs were found in the coprolites [26]. The 6th case was another example of ectopic paragonimiasis in the liver (well-demarcated mass) in a 17th-century male mummy at Cheongdo (Fig. 7), in which case the eggs (av. 84.0×51.5 μm) were found from the inner-trabecular material of the liver mass [102]. The 7th–9th mummy cases were reported by Hong et al. [103]; the mummies were discovered at Hwasung (eggs were found in the feces from the intestines, dating to the 18th century) and Yeounggwang-1/Yeounggwang-2 (found in the organic materials compiled upon the hipbones of 2 mummies dating to the 15th–16th century) and molecular studies were done. The 10th case was a female mummy excavated at Wonju dating to the 16th–17th century, and P. westermani (av. 84.8× 46.1 μm in length) eggs were detected in the coprolites [35]. The recovery of P. westermani eggs in archaeological soil specimens was reported once by Cho et al. [30] (Table 5). The soil was sampled from the outlet of a presumed toilet near an ancient house in Seoul dating to the 15th century [29].
Molecular studiesMolecular studies on P. westermani eggs were performed in 7 of the 10 mummy specimens examined in Korea [101,103]. Shin et al. [101] used ITS2 gene sequences obtained from the eggs detected in an ectopic paragonimiasis case (female mummy) in the liver of Hadong-2 mummy and found 97–100% identity with those of P. westermani available in GenBank. Hong et al. [103] analyzed ITS2 and cox1 sequences from the eggs detected in 6 mummies (Yongin, Dangjin, Cheongdo, Hwasung, Yeounggwang-1, and Yeounggwang-2) and found 95–100% identity with P. westermani for ITS2 and 84–100% identity for cox1. It was suggested that ITS2 is superior to cox1 in discriminating P. westermani from other Paragonimus spp. [103].
World reports and evolutionary aspects of Paragonimus spp.It is assumed that some human parasites, like Paragonimus, were spread around the planet with the migration of humans out of Africa during the last 100,000 years [104]. Humans might have acquired Paragonimus spp. infection during their migration as a souvenir parasite (possibly in Asia), since this infection has not been found in ancient African human populations [64,104]. Paragonimus spp. infecting humans may have come from carnivorous mammals, including tigers, leopards, lions, skunks, and hominid ancestors. Thus, the phylogenetic origin of Paragonimus spp. infecting animals may be older than 100,000 years. Among the human infecting Paragonimus spp., P. westermani may be the oldest one having the widest geographical distribution, and P. skrjabini, P. skrjabini miyazakii, and P. heterotremus, may have split from P. westermani somewhere in Asia (China, Japan, and Indochina peninsula). Without evidence, P. kellicotti and P. mexicanus are considered to have split from P. westermani during human migration (transberingeal or transoceanic) to the North and South Americas, respectively. Whether P. africanus, P. uterobilateralis, and P. gondwanensis are antecedents of P. westermani or vice versa needs further investigation.
The oldest Paragonimus sp. eggs ever found in human remains were those (probably Paragonimus caliensis) detected in human coprolites from the Atacama Desert of northern Chile dating to 5900 BCE [95]. The next oldest Paragonimus sp. (possibly P. westermani) eggs were those detected in a large amount in the soil samples from a complete toilet at the Makimuku site, Sakurai City, Nara Prefecture, Japan dating to the early 3rd century [90]. The 3rd discovery of Paragonimus (as P. westermani) eggs was reported by Shin et al. in 2009 [18] from a mummy at Gongju in Korea. P. caliensis (non-human infecting Paragonimus species) was often considered a morphological variant of P. mexicanus (human-infecting species), but recently P. caliensis was recognized as a distinct species based on morphological and molecular analyses [105].
Metagonimus yokogawai and other Metagonimus spp.General viewsSix species of Metagonimus (Digenea: Heterophyidae), namely, M. yokogawai, M. miyatai, M. takahashii, M. suifunensis, M. minutus, and M. katsuradai, have been known to infect humans [80,83]. These species are distributed mainly in Far Eastern Asia, including Korea, Japan, China, Taiwan, and Russia [80,83,85]. The mode of human infection is the consumption of raw or improperly cooked freshwater fish, such as sweet smelt (formerly sweetfish; Plecoglossus altivelis), chubs (Zacco platypus, Z. koreanus, Z. temminckii, etc.), or carps (Carrasius auratus, Cyprinus carpio, etc.) [80,83,85]. Patients infected with Metagonimus spp. may experience abdominal discomfort, diarrhea, indigestion, and lethargy [80,83].
Paleoparasitological studies on Metagonimus spp. in KoreaIn Korea, the ancient M. yokogawai eggs were detected in 4 mummies and 1 archaeological soil sample during 2008–2021 (Table 6) [7,26,30,33,40]. The first discovery was documented by Seo et al. in 2008 [7] from the feces of a mummy at Hadong-1 dating to the 16th-early 17th century. The egg size was 30.8±1.8×16.9±0.9 μm [7]. Hadong County is located at the mouth of the Seomjin River and has been until currently one of the highest endemic areas of M. yokogawai infection [7]. The second discovery of M. yokogawai eggs was in the feces and soil of a mummy found at Sapgyo dating to the 16th century [40]. The egg size, in this case, was 31.2±0.7×15.4±0.1 μm [40], slightly larger than those of the Hadong mummy, resembling M. miyatai eggs. Later, the eggs obtained from this mummy were molecularly analyzed using the 28S rDNA sequence (sequencing of the cox1 gene was done but not successful), and the identity with those of M. yokogawai (Korea and Japan) in GenBank was 98.8–100%, whereas its identities with M. miyatai and M. takahashii were 99.6% and 99.2%, respectively [106]. Thus, it was difficult to determine the Metagonimus sp. of the Sapgyo mummy, and the usefulness of the 28S rDNA gene for the molecular discrimination of Metagonimus spp. was questioned [106]. To overcome this difficulty, the cox1 gene sequence should be analyzed, as this gene locus was better for proper discrimination of Metagonimus spp. [107]. Sapgyo Town (Chungcheongnam-do) is currently considered an endemic area of M. miyatai, and its adjacent areas along the Daecheong Reservoir and Geum River (Chungcheongnam-do) were reported to be, since the 1980s, endemic areas of M. miyatai [108,109]. However, it should be ruled out that the life cycle of M. yokogawai might have also existed in the Sapgyo area several hundred years ago. One of the important ecological factors is whether sweet smelt (Plecoglossus altivelis) were available along the Sapgyo stream at that time, the most potential fish host for M. yokogawai [80,83,85]. The 3rd report on the detection of M. yokogawai eggs (Fig. 8A) in Korea was in the coprolites of a mummy at Sacheon (Fig. 1, no. 42) dating to 1620–1630 CE; the average egg size was 27.5× 15.6 μm [26]. Sacheon County is located slightly southeast of Hadong County, one of the highest endemic areas of M. yokogawai. Later, M. yokogawai eggs (29–30×15 μm) were found again in the soil from the yard of an old house in old Seoul City dating to the 15th century [30], which is the 4th discovery. This house seemed to have been owned by an upper-class government authority who occasionally enjoyed eating raw freshwater fish (i.e., sweet smelt). The 5th discovery of Metagonimus sp. eggs (27.5±0.0×16.3±1.2 μm), probably M. yokogawai, was from the hip bone precipitates of a mummy at Goryeong dating to the 17th century [33].
Molecular studiesMolecular studies of M. yokogawai were done by Hong et al. [106] using the eggs obtained from 3 mummies in Hadong-1, Sapgyo, and Sacheon [7,26,40] and by Oh et al. [107] using the eggs obtained from a Goryeong mummy [33]. The 28S rRNA and cox1 genes were analyzed (not successful in Sacheon mummy), and the results revealed that the cox1 gene was better than the 28S rRNA gene for discrimination of Metagonimus spp. [106,107]. The eggs from the Hadong-1 mummy were identified as those of M. yokogawai based on cox1 sequences, with 96.6–99.7% and 99.7–100% homologies with the sequences reported from Korea and Japan, respectively [106]. Meanwhile, the cox1 sequence identities of the eggs from the Hadong-1 mummy with M. miyatai and M. takahashii deposited in GenBank were only 87.4% and 86.2%, respectively [106]. Regarding the Goryeong mummy, the cox1 sequence identity with M. yokogawai was 99.7–100%, whereas its identity with M. miyatai and M. takahashii was 86.7–86.9% and 86.1–86.7%, respectively, confirming its specific diagnosis of M. yokogawai [107]. However, regarding the eggs of the Sapgyo mummy, sequencing was successful only in 28S rDNA, and its homologies with 28 rRNA sequences of M. yokogawai, M. miyatai, and M. takahashii were all over 99.0%, so it was difficult to finalize the specific diagnosis [106]. Considering the slightly large egg size and the contemporary geographical distribution of M. miyatai, M. yokogawai, and M. takahashii, it is cautiously suggested that the eggs of the Sapgyo mummy could be those of M. miyatai.
World reports and evolutionary aspects of Metagonimus spp.The oldest M. yokogawai eggs ever recorded were those detected in the soil sample from toilets at the Fujiwara Capital, Heijo Capital, and the Ko-ro-kan in Dazaifu, Nara Prefecture, Japan dating to the 7th–8th century [90]. The next oldest M. yokogawai eggs were those found in Korea from the soil sediments from the yard of an old house at Seoul dating to the 15th century [30], followed by those found in the mummy feces or coprolites excavated at Sapgyo (16th century) [40], Hadong-1 (16th-early 17th century) [7], Sacheon (1620–1630 CE) [26], and Goryeong (17th century) [33].
The evolutionary origin of M. yokogawai is difficult to assess. The morphology, as well as the biology, of M. yokogawai are highly like those of C. sinensis. The only remarkable difference is the habitat in the definitive host; M. yokogawai parasitizes the intestine (phylogenetically older), whereas C. sinensis infects the bile duct (phylogenetically younger). Based on molecular analysis of 18S rRNA and ITS2 genes, the families Opisthorchiidae and Heterophyidae were found to be phylogenetically very close and almost inseparable from one another, with the former nested within the latter [110]. Thus, it could be speculated that the evolutionary age of M. yokogawai and heterophyid flukes might be older (>3 million years) than that of the small liver flukes, including C. sinensis, O. felineus, and O. viverrini.
Pygidiopsis summa and other heterophyid trematodesGeneral views
Pygidiopsis summa (Digenea: Heterophyidae) is an intestinal trematode of humans and animals in Korea, Japan, and Vietnam [80,83]. This fluke is contracted to humans by the ingestion of raw or undercooked brackish water fish (estuary fish), including mullet, perch, and goby [83,85]. Patients infected with P. summa may experience clinical symptoms, such as abdominal pain, diarrhea, indigestion, and lethargy [80,83]. Pygidiopsis genata is another species infecting humans in Egypt, the Middle East, the Philippines, Tunisia, and Ukraine [80].
Paleoparasitological studies on Pygidiopsis summa in Korea
Pygidiopsis summa eggs (Fig. 8B) were detected one time in 2016 in a strata soil sample excavated at Buyeo-2 dating to the 5th–6th century of the Baekje Dynasty (Table 6) [111]. Buyeo was the capital city and the political and cultural center of the Baekje Kingdom, where plenty of people gathered [111]. Nowadays, P. summa is distributed mainly along the western coastal areas, including the estuary villages of Chungcheongnam-do, Jeollabuk-do, and Jeollanam-do.
World reports and evolutionary aspects of P. summa and heterophyid flukesIt could be speculated that the evolutionary age of P. summa and other heterophyid flukes might be older (>3 million years) than that of the small liver flukes, including C. sinensis, O. felineus, and O. viverrini, which might have been split from heterophyid flukes, including M. yokogawai, P. summa, and their ancestors.
The eggs of Cryptocotyle lingua, a parasite of fish and very rarely of humans [83], were found in the frozen body of a female Eskimo mummy on St. Lawrence Island, Bering Sea (Alaska, USA) dating to 405±70 CE [112]. However, this mummy case was considered to have been a false parasitism [112]. Additionally, a fluke egg, 35×18 μm in size, presumed to be of Heterophyes, Opisthorchis, and Clonorchis, was found in the coprolite (probably of human origin) from Glen Canyon, Utah, USA dating to 1250 CE [94]; however, it was also considered a spurious infection [95].
Gymnophalloides seoiGeneral views
Gynophalloides seoi (Digenea: Gymnophallidae) was reported as a human-infecting intestinal fluke in 1993 in Korea [113,114]. The endemic areas are scattered in coastal areas of Shinan-gun (a southwestern part of Korea), and among them, Aphae-do (Island) is the highest endemic area [113,114]. In heavy infections, G. seoi may cause gastrointestinal troubles, indigestion, and occasionally symptoms like diabetes [114]. The mode of human infections is eating natural oysters (Crassostrea gigas) raw or under improperly cooked conditions; however, cultivated oysters have been found free from infection [113,114]. The natural definitive host is waders, including oystercatchers, and mammals, such as cats [85,114].
Paleoparasitological studies on Gymnophalloides seoi in Korea
Gynophalloides seoi eggs were detected two times in the feces or soil sediment of two mummy specimens excavated at Hadong-1 and Sapgyo (Table 6) [7,40]. The first discovery of these eggs (20.0±1.7×12.0±0.7 μm) was from the feces (luminal surface of the rectum) of a female mummy at Hadong-1 dating to the 16th-early 17th century [7]. However, the egg count was remarkably high, showing an average value of eggs per gram of feces (EPG) of 21,417 in 6 times repeated measurements [7]. This finding was surprising and interesting in several points. First, this discovery indicated that G. seoi might have been prevalent in the Hadong area, which is remote from the current major endemic area (i.e., Shinan-gun) [113, 114]. Thus, it was speculated that G. seoi might have occurred widely along the western and southern coastal areas of the Korean peninsula several hundred years ago [7]. Second, the worm load of G. seoi in this mummy is estimated to be extremely high, ca. 93,117 worms, based on the report that the average EPG/worm in human infections was 0.23 [114]. This figure of individual worm load appears to be the highest of all published data in Korea [114]. Third, it is suggested that the people at that time liked to eat raw oysters. The second report on the recovery of G. seoi eggs (19.9±2.5×12.1±0.8 μm) (Fig. 9A, B) was from the fecal-soil samples on the pelvic bone of a male mummy at Sapgyo dating to the 16th century [40]. The EPG was 150.8 [40], and the worm load was estimated to be at about 657 worms. Sapgyo area is in the middle of the western coastal area which is remote from Shinan-gun, and the discovery of this mummy case here supported the speculation that G. seoi infection may have occurred widely along the western and southern coastal areas of the Korean peninsula [113,114].
Evolutionary aspects of Gymnophalloides seoiThe evolutionary origin of G. seoi is difficult to estimate because of the lack of available data. Probably it was an intestinal/bile duct fluke of wading birds transmitted by oysters for a long time [113,114], and it may have become capable of infecting humans at a certain time point. The evolutionary age of G. seoi might be older (>3 million years) than that of the liver flukes, including C. sinensis, O. felineus, and O. viverrini. Molecular studies on Gymnophallidae flukes and other related families may help the phylogeny and evolution of G. seoi and related gymnophallid fluke species.
Isthmiophora hortensis and other echinostome speciesGeneral viewsAt least 23 echinostome species (8 genera) are currently recognized as human-infecting species worldwide [83]. Echinostoma, Isthmiophora, Echinochasmus, Acanthoparyphium, Artyfechinostomum, Echinoparyphium, Himasthla, and Hypoderaeum are the responsible genera [83]. They are intestinal flukes of fish, reptiles, birds, and mammals, including humans [83]. Human infections occur mainly through the consumption of infected snails, clams, or fish [83]. In the genus Isthmiophora, two species, namely I. hortensis and I. melis, are known to cause human infections [83].
Isthmiophora hortensis (syn. Echinostoma hortense) (Digenea: Echinostomatidae) is an epidemiologically important echinostome fluke infecting the intestinal tract of humans and animals, including dogs, cats, and rats in Korea, Japan, and China [80,83,85]. The source of infection is freshwater fish, including the loach (Misgurnus anguillicaudatus), the Korean spotted sleeper (Odontobutis obscura interrupta), and the fat minnow (Moroco oxycephalus) [80,83,85]. In human infections, this echinostome can cause abdominal pain and diarrhea, and the adult fluke has been occasionally found attached to the ulcerated mucosa in the duodenum or upper jejunum of patients during gastroduodenal endoscopy [80,83,85]. In some endemic areas in Korea, the prevalence among the human population was high, up to 22.4%, in 1988 [85].
Paleoparasitological studies on Isthmiophora hortensis in Korea
Isthmiophora hortensis eggs were discovered one time in an archaeological strata soil specimen from an old house at Buyeo-2 of Baekje Capital ruins dating to the 6th–7th century [32] (Table 6). The eggs were large, dark brown, operculated, immature, and 112.5–125.0 μm in length and 62.5–67.5 μm in width [32]. The number of I. hortensis eggs in 1 g of the soil sample was 210. This soil sample was obtained from an old house; but it was uncertain whether the soil sample was of human or animal (dog, cat, or rat) origin [32].
World reports and evolutionary aspects of echinostome flukesEggs of Echinostoma sp. were first detected in the coprolite of an adult male mummy from a cave in Lapa do Boquete in Minas Gerais State, Brazil dating to 560±40 years BP [115,116]. Molecular analysis of this case revealed that, in the ITS1 region, the eggs were identical to both E. paraensei and E. luisreyi; however, in the mitochondrial cox1 gene, they were more compatible with E. paraensei [116]. At least 4 more reports have been published on echinostome infections in archaeological remains. Two of them were reported from human remains in Mexico [117] and Belgium [118], one from humans or animals in Korea [32], and one from felid coprolites in Brazil [119]. The Mexican report was on the discovery of Echinostoma sp. eggs (av. 108×66 μm) in 3 of 36 coprolites (human origin) dating to about 1,400 years BP from the Cueva de Los Muertos Chiquitos, Rio Zape Valley, Durango, Mexico; the eggs were suspected as those of E. revolutum [117]. The report from Belgium was the discovery of Echinostoma sp. eggs (86–116×58–69 μm) in 8 of 14 sediment samples from 7 cesspits (human origin) dating to 1100–1600 CE in the city of Aalst near the eastern border of the County of Flanders, Belgium [118]. Echinostome eggs (Echinostoma sp.) were detected in felid coprolites (96 units identified as belonging to the group of felines) dating from 1,040±50 to 1,730±70 BP from the Furna do Estrago Archaeological Site, Pernambuco State, Brazil, which measured 70.0–97.5×35.0–62.5 μm [119].
Echinostome flukes are primarily intestinal parasites of birds and, to a lesser extent, mammals, including humans [83]. The evolutionary age of echinostomes might be as old as that of the large liver flukes, F. hepatica and F. gigantica (5.3–10 million years) [71,72] and might be older than that of the small liver flukes (>3 million years). Among the zoonotic echinostome species, the phylogenetic age of I. hortensis might be younger than the other species in the same family, since I. hortensis is exclusively a parasite of mammals, whereas Echinostoma revolutum, Hypoderaeum conoideum, and many other echinostome species are primarily avian parasites [83]. I. hortensis might have split from a certain species of avian echinostomes by changing the host. Molecular studies on echinostomes may help the phylogeny and evolution of I. hortensis and other echinostome species.
Dibothriocephalus nihonkaiensis and other diphyllobothriid tapewormsGeneral views
Dibothriocephalus nihonkaiensis (syn. Diphyllobothrium nihonkaiense), Dibothriocephalus latus (syn. Diphyllobothrium latum), and Adenocephalus pacificus (syn. Diphyllobothrium pacificum) are 3 major broad fish tapeworms (Eucestoda: Diphyllobothriidea) infecting humans worldwide [120]. D. nihonkaiensis is distributed in Japan, Korea, Russia, and Europe, and D. latus is distributed mainly in Europe and North America [120,121]. A. pacificus has been reported in North (the USA) and South America (Chile and Peru) [120,121]. These dibothriocephalid tapeworms are contracted to humans by the consumption of raw or undercooked salmon, perch, pike, trout, or other kinds of freshwater or brackish water fish [13]. Natural definitive hosts include dogs, cats, foxes, leopards, bears, and seals [13]. Most infected humans complain of almost no or mild gastrointestinal discomfort but in a few instances, significant mass effect (gut obstruction) and pernicious (vitamin B12 deficiency) anemia may develop [13].
Paleoparasitological studies on Dibothriocephalus nihonkaiensis in KoreaOne article has been published on the discovery of probable D. nihonkaiensis eggs (50–65 ×37.5–47.5 μm) under the name Diphyllobothrium latum in an archaeological soil sample from the Royal Arsenal in Seoul dating to the 15th–16th century [22] (Table 7). In mummy samples, dibothriocephalid eggs have never been found in Korea. Before 2009, in Korea, the species name used for Diphyllobothrium tapeworms was usually D. latum, but it was changed to Diphyllobothrium nihonkaiense after a molecular study [122,123]. In the meantime, in 2017, it was suggested that the generic name of Diphyllobothrium latum and Diphyllobothrium nihonkaiense should be renamed as Dibothriocephalus [120]. Therefore, their species names have been resurrected/revised as Dibothriocephalus latus and Dibothriocephalus nihonkaiensis, respectively [120]. We would like to accept this taxonomic move. The soil sample examined in the Korean report was taken from a military facility of the Joseon Dynasty [22]. It is speculated that certain people who worked in the military of the Joseon Dynasty enjoyed raw or improperly cooked salmon, perch, or trout.
World reports and evolutionary aspects of dibothriocephalid tapewormsDibothriocephalid tapeworm eggs have been discovered frequently in archaeological specimens in Europe and America but rarely in Asia, Africa, and the Middle East [39,121,124–126]. The first discovery of dibothriocephalid eggs (named Bothriocephalus latus; currently Dibothriocephalus latus) was reported by Szidat in 1944; the eggs were found in the intestinal content of a bog body (man) from Prussia dating to 1,500 years BP [2]. The oldest dibothriocephalid eggs ever recorded were those discovered in archaic coprolites from the Pacific Coasts of Peru where ancient people lived from 4,000 to 10,000 years BP and ate large quantities of fish [39]. The eggs were assigned as those of A. pacificus (under the name Diphyllobothrium pacificum). Similar old dibothriocephalid (reported as Diphyllobothrium sp.) eggs were discovered in the soil sediment from burials in the Shillourokambos Site in Cyprus dating to 9,500–9,600 BP [124,127]. Thereafter, numerous reports have been published on the recovery of dibothriocephalid eggs in archaeological remains around the world [124,125]. Notably, from 1989–2010, at least 20 records have been gathered by Reims Paleoparasitology Laboratory in France, regarding the recovery of Diphyllobothrium sp. eggs in archaeological materials from various European (France, Germany, Switzerland, and Cyprus), Middle East (Israel), and African (Egypt and Sudan) countries [125]. In Asia, several articles have been published regarding the recovery of dibothriocephalid eggs from human archaeological remains, including 1 article from Korea [22], 1 from Japan [90], and at least 4 articles from Russia [77]. In Japan, Diphyllobothrium sp. (probably D. nihonkaiensis) eggs were discovered in cesspit toilets at Yanagi-no-gosho Site in Hiraizumi Town, Iwate Prefecture (Iwate Prefectural Archaeological Center) dating to the 12th century [90]. In Russia, D. latus (under the name D. latum) infection was discovered in human or animal remains (pelvic soil/coprolites) excavated in Western and Eastern Siberia [77,128].
Regarding the higher level eucestodes (subclass Eucestoda), the early diversification may have been associated with deep co-speciation with vertebrate taxa, extending into the Palaeozoic era, minimally 350–420 million years ago [129]. It seems probable that the current human-infecting dibothriocephalid tapeworms have evolved from those infecting carnivorous animals, and it may have occurred around the time (for example, 400,000–600,000 years BP; Late Neolithic Era) when humans began to consume fish as one of their main diets. A. pacificus may have evolved separately from D. latus and D. nihonkaiensis, and the latter two species may have evolved from their common ancestors.
Taenia tapewormsGeneral viewsWithin the genus Taenia, 3 important species are known to occur in humans, namely, T. solium, T. saginata, and T. asiatica [13,130]. Their adult stages can cause intestinal infections in humans (exclusive definitive host), and the larval stage (=metacestode) of 1 species, T. solium, can cause human infections (cysticercosis) in subcutaneous tissues, muscles, and visceral organs, including the brain [13]. T. solium and T. asiatica are transmitted by the consumption of pork, whereas T. saginata is transmitted by eating beef [13,130]. The geographical distribution of T. solium and T. saginata is almost worldwide (Asia, Europe, Africa, the Middle East, and the Americas), where pigs and/or cattle are raised, except in areas where pork or beef are not eaten because of religious reasons [13]. The distribution of T. asiatica is somewhat limited to southeast Asia, including China, Taiwan, Thailand, Lao PDR, Nepal, Bangladesh, India, Indonesia, the Philippines, Korea, Japan, and Vietnam [130]. In most human infections, intestinal taeniases may elicit no special clinical symptoms, except for mild gastrointestinal discomfort and occasional discharge of a part of proglottids in the feces [13]. However, in human cysticercosis, mild to severe clinical outcomes may occur [13]. An important problem in the diagnosis of taeniid eggs in archaeological samples includes high morphological similarity between species of Taenia (egg size 31–43 μm in diameter) and Echinococccus (egg size 32–36×25–30 μm) except for slight differences in egg size, and it is practically difficult to provide an ultimate (specific) diagnosis only by egg morphology [13,42].
Paleoparasitological studies on Taenia spp. in KoreaThe eggs of taeniid tapeworms have been discovered 3 times in archaeological specimens (Table 7) [28,29,131]. The first one was the recovery of taeniid eggs (Fig. 10A) in the mummy soil (hip bones and sacrum) from a 17th-century tomb excavated in Gongju [131]. In the first examination of this mummy sample, taeniid eggs were not found [18]. However, in the second examination, Taenia sp. eggs (37.5–40.0×37.5 μm), 5 in number, were discovered [131]. The eggs revealed characteristic features with a radially striated embryophore (eggshell), although 6 hooklets inside the embryo (seen in well-prepared modern fecal samples; Fig. 10B) were not clearly visible. In Korea, all 3 species of Taenia have likely existed for a long time, and thus the specific diagnosis was not possible. The second discovery of taeniid eggs (35.0–39.8×31.0–38.6 μm) was in the soil sediment samples (2 strata samples) from archaeological sites in Changnyeong (Hwawang Sansung; a mountaintop fortress reservoir) of the ancient Silla Kingdom dating to ~676 CE [28]. As the number of eggs deposited in the environment by an adult Taenia sp. tapeworm is relatively small compared to other species of helminths [13], the recovery of quite many Taenia spp. eggs (0.4–0.5 EPG of soil sample) in a fortress reservoir was considered unusual [28]. It was speculated that human taeniases were quite prevalent at that time, and human or animal waste scattered in and around the fortress was swept via streams into the reservoir, as it was situated at a relatively low altitude [28]. The third recovery of taeniid eggs (32.5 μm in diameter) was in the coprolites of a male mummy (estimated to be 25–29 years old) from Seoul (Junggye) dating to the 16th–17th century [29].
World reports and evolutionary aspects of Taenia spp.The oldest Taenia spp. eggs ever recorded from archaeological remains around the world were those found in 2 localities (Shillourokambos and Khirokitia) of Cyprus [127]. In the Shillourokambos Site, Taenia eggs were found in soil sediments dating to 9,500–10,500 years BP [127]. In the Khirokitia Site, Taenia eggs were discovered in soil sediments from a burial dating to 6,590±260 years BP [127]. The next oldest taeniid eggs were those found in human coprolites from Hogup Cave, Utah, USA dating to 6,200–6,500 years BP [124,132]. The third oldest taeniid eggs were those found in coprolites of probable human origin in 4 places (Arbon-Bleiche 3, Sipplingen, Torwiesen II, and Walhausen-Ziegelhütte) of the Horgen cultural site in Switzerland dating to 5,050–5,384 years BP [133]. Numerous other reports have been published on the discovery of taeniid eggs in archaeological materials [124], including a report from Japan [90].
It was a general concept that the evolutionary origin of the human infecting Taenia tapeworms (T. solium, T. saginata, and T. asiatica) was relatively recent, not more than 10,000 years BP, coincidental with the domestication of major food animals, including swine and cattle, and the advent of agriculture [130]. However, a new hypothesis was proposed based on phylogenetic analyses that the origin of the human infecting Taenia tapeworms may have preceded the domestication of cattle and swine [134]. Hominids, on the savannah of Africa, became hosts for Taenia before the origin of modern humans and substantially earlier than the domestication of bovids and suids and the development of agriculture [134]. African hominids that scavenged or preyed upon antelope and other bovids were exposed to colonization by Taenia tapeworms that were using hyaenids, canids, and felids as definitive hosts and bovids as intermediate hosts [134]. T. saginata and T. asiatica may have diverged from their common ancestor at least 90,000–160,000 years BP or from 0.78–1.71 Myr BP depending on the methods of analyses [134]. Phylogenetically, T. saginata and T. asiatica are sister species, with both being distantly related to T. solium [129]. T. saginata + T. asiatica and T. solium represent the results of 2 independent host shifts to hominids [134]. The divergence of T. asiatica from its ancestor may have been a consequence of the dispersal of hominids from Africa to Asia with the subsequent isolation of hosts and parasites [134].
Echinococcus granulosusGeneral views
Echinococcus granulosus and E. multilocularis are 2 important Echinococcus species infecting humans [13]. Their life cycles start from the eggs which are liberated from the adult worms in the intestines of dogs, cats, wolves, foxes, jackals, dingoes, hyenas, pumas, and jaguars [13]. When the eggs (already embryonated in the worm uterus) contaminated in the environment are eaten by herbivorous mammals (sheep, goats, camels, hogs, and oxen) or humans, they develop into the larval stage, i.e., hydatid cyst, in various organs and tissues of these intermediate hosts [13]. If the definitive host eats the flesh of these animals, the hydatid cysts grow to be adults in the intestine [13]. Humans are an accidental host for these cestodes, and they suffer from hydatidosis in the liver, lungs, and brain in endemic areas [13].
World reports and evolutionary aspects of Echinococcus granulosusIn Korea, hydatid cysts have never been discovered in archaeological human remains. However, hydatid cysts of E. granulosus have been found frequently in Europe [39,135], the USA [46], the Middle East (Israel) [46,125], and Egypt [46]. In Europe, calcified hydatid cysts have been found in archaeological human remains; about 25 cases from 18 different sites dating from Hellenistic to post-medieval periods [135]. The countries where old hydatid cysts were reported included Italy, France, Switzerland, Spain, Greece, Denmark, Iceland, Poland, Ireland, England, the USA, and Scotland [39,135]. The oldest hydatid cyst ever reported seems to be the one discovered in mummies from Thebes, Egypt dating to 750-525 BCE [39,136]. The next oldest was the one reported from the burial sites in Mt. Scopus, Jerusalem, Israel dating to 538 BCE [39], followed by a report from Schulz Site, Michigan, the USA dating to 300 BCE [39] and from Orton Longueville, Cambridgeshire, England dating to the 2nd century BCE [135].
The host-parasite relationships of E. granulosus were probably well established in the present form by the late Pleistocene (ca. 127,000 years BP), and conditions favoring its sylvatic cycle appear to have existed in Europe since early Neolithic time (ca. 10,000 years BP) with the original wolf-ungulate host relationship gradually being replaced by, and coexisting, with the dog-domestic ungulate relationship [137]. As early as 870 CE, it was introduced into Iceland and became endemic there by 1200 CE; infected dogs then probably transported it to western Europe, North Africa, and the Mediterranean region via whaling ship [137]. It may have been carried into North America by Norseman who colonized Newfoundland more than 1,000 years BP, of which the life cycle was established in eastern North America by the 17th century [137]. In South America, E. granulosus life cycle was established probably by the mid-1500s CE when the continent was colonized by Spaniards and their dogs [137]. By 1900 CE, E. granulosus was present throughout the livestock-raising regions of Australia and New Zealand [137].
Estimation of the prevalence of helminthic infections among the medieval Korean populationSoil-transmitted helminthsUsing the mummy study data, Seo et al. [29] and Oh et al. [35] tried to estimate the prevalence of helminthic infections (by species) among the pre-modern Korean populations. The prevalence of overall helminth species in mummy samples was 100% (30/30), and those of A. lumbricoides and T. trichiura were 56.7% (17/30) and 86.7% (26/30), respectively [35]. These figures could be compared with the contemporary Korean report covering from 1971 to 2012 [138]. In 1971, the prevalence of overall helminths, A. lumbricoides, and T. trichiura was 84.3%, 54.9%, and 65.4% [138], respectively, which were highly correlated with the figures from the Joseon mummies [35]. However, after the 1990s, the contemporary status of these soil-transmitted helminths has been very much improved in Korea, and the prevalence became almost negligible after 2012 (0.03% and 0.41% for Ascaris and Trichuris, respectively, in the prevalence among the general population) [138].
Trematode infectionsSeo et al. [29] and Oh et al. [35] also tried to estimate the prevalence of trematode infections among the medieval Korean population by using mummy study data. The prevalence of C. sinensis infection in mummy samples was calculated to be 30.0% (9/30) as of 2023 [35]. The contemporary report on the general Korean population revealed that the prevalence of C. sinensis was 4.6% (in 1971), 2.6% (1981), 2.2% (1992), 2.4% (2004), and 1.9% (2012) [138]. The prevalence appeared to be much higher in medieval populations than in the modern Korean population. As C. sinensis infection occurs through the consumption of raw freshwater fish, it could be speculated that middle-to-high-class Korean people (to which most of the Joseon mummy cases belonged) enjoyed eating raw freshwater fish. In old Korean literature, it is written that the raw flesh of common carp (Cyprinus carpio) and crusian carp (C. auratus) was frequently served in gatherings of noble Korean societies [139].
The prevalence of P. westermani infection in Korean mummies was calculated to be 30.0% (9/30 mummies) as of 2023 [35,40]. This prevalence was very high compared to the contemporary status of P. westermani infection among the general Korean population [138]. Notably, however, in 1884, Dr. Allen H.N., an American physician who volunteered in Korea (Joseon Kingdom) as a Presbyterian missionary, reported 2 years later that most hemoptysis patients in Korea were due to infection with ‘distoma’ (lung fluke, probably P. westermani) [141]. In 1925, the prevalence of P. westermani infection among the Korean population was estimated to be about 9.4% (16,866 out of 180,351) by the egg detection method in the sputum [141]. However, the national prevalence of paragonimiasis in Korea decreased remarkably to lower than 0.1% nowadays (personal communication).
The average prevalence of M. yokogawai (and possibly M. miyatai and M. takahashii) infection in the Joseon mummies could be calculated roughly as 13.3% (4/30). This rate is much higher than the contemporary status in Korea; 1.2% in 1981, 1.0% in 1986, and 0.26–0.5% in 1992–2012 [138]. Thus, the prevalence of M. yokogawai infection in mummy samples was more than 10 times higher than the contemporary figures. One of the reasons may include that most of the mummy cases were middle-to-high-class Joseon Dynasty people who favored eating raw freshwater fish.
Taenia spp.Based on the data from Korean mummies until 2023, the average prevalence of Taenia spp. infection in the Joseon mummies was calculated as 6.7% (2/30). This rate is quite comparable with the 1920’s Korean reports of human taeniases (6–16%) [142]. However, the prevalence became remarkably lower based on fecal examinations of the general population in Korea, 0.7–1.9% in the 1970s, 0.3–1.1% in the 1980s, 0.02–0.06% in the 1990s, and 0.00–0.04% in 2004–2012 [138]. It has been suggested that the Joseon people may have been infected with T. saginata more frequently than T. asiatica, since the consumption of beef was quite common among the Joseon people, and pork was not consumed as much as we thought [142].
Issues and PerspectivesIn this review, we introduced and discussed the major paleoparasitological findings reported by Korean paleoparasitologists during the past 26 years (1997–2023). The findings were mostly on helminth eggs and larvae detected in archaeological remains, including human mummies and soil sediment samples. The covered period of the ancient remains ranged from the Three Kingdoms’ Period (100 BCE) to the Joseon Dynasty (~1900 CE). However, searching for more ancient human and/or animal remains is strongly recommended. We also regret that we could not find any referable paleoparasitological report regarding protozoan parasites, including Plasmodium spp., Toxoplasma, and intestinal amoebae in Korea. Regarding arthropod parasites, including ticks, mites, mosquitoes, lice, and bugs, there have been a few archaeological reports in Korea, and it is hoped that the major findings will be reviewed and introduced soon. Molecular techniques have been applied to analyze the aDNA of helminth eggs in Korea, including A. lumbricoides, T. trichiura, C. sinensis, P. westermani, and M. yokogawai. However, applying molecular techniques should be extended to other helminth parasites, for example, heterophyids (other than M. yokogawai), G. seoi, echinostomes, dibothriocephalids, and Taenia tapeworms, as well as protozoan and arthropod parasites.
AcknowledgmentsWe are grateful to our colleagues in the Department of Anatomy and Forensic Science, Seoul National University College of Medicine, Seoul, Korea, and the Department of Parasitology, Dankook University College of Medicine, Cheonan, Korea, who cooperated with us in mummy studies and other archaeological research. We would like to extend our sincere appreciation to the Korean archaeologists and paleopathologists who co-worked with us.
NotesAuthor contributions
Conceptualization: Chai JY, Shin DH, Seo M
Data curation: Chai JY, Shin DH, Seo M
Formal analysis: Chai JY, Shin DH, Seo M
Investigation: Chai JY, Shin DH, Seo M
Methodology: Chai JY, Shin DH, Seo M
Project administration: Chai JY, Shin DH
Resources: Chai JY, Shin DH, Seo M
Software: Chai JY, Shin DH, Seo M
Supervision: Chai JY
Validation: Chai JY, Shin DH, Seo M
Visualization: Chai JY, Shin DH, Seo M
Writing-original draft: Chai JY
Writing-reviews & editing: Chai JY, Shin DH, Seo M
Conflict of interestThe authors declare that there are no conflicts of interest related to this study.
References1. Ruffer MA. Note on the presence of ‘Bilharzia haematobia’ in Egyptian mummies of the twentieth Dynasty. Brit Med J 1910;1(2557):16
https://doi.org/10.1136/bmj.1.2557.16-a
2. Szidat L. Über die Erhaltungsfähigkeit von Helmintheneiern in vor- und Frühgeschichtlichen Moorleichen. Z Parasitenkd 1944;13:265-274
https://doi.org/10.1007/bf03177148
3. Seo M, Araujo A, Reinhard K, Chai JY, Shin DH. Paleoparasitological studies on mummies of the Joseon Dynasty, Korea. Korean J Parasitol 2014;52(3):235-242
https://doi.org/10.3347/kjp.2014.52.3.235
4. Kwangju National Museum. Shinchang-dong wetland site I. Report on the Research of Antiquities of the Kwangju National Museum. Kwangju National Museum 1997;33:159-166 (in Korean).
5. Han ET, Guk SM, Kim JL, Jeong HJ, Kim SN, et al. Detection of parasite eggs from archaeological excavations in the Republic of Korea. Mem Inst Oswaldo Cruz 2003;98(suppl 1):123-126
https://doi.org/10.1590/S0074-02762003000900018
6. Seo M, Guk SM, Kim J, Chai JY, Bok GD, et al. Paleoparasitological report on the stool from a medieval child mummy in Yangju, Korea. J Parasitol 2007;93(3):589-592
https://doi.org/10.1645/GE-905R3.1
7. Seo M, Shin DH, Guk SM, Oh CS, Lee EJ, et al.
Gymnophalloides seoi eggs from the stool of a 17th century female mummy found in Hadong, Republic of Korea. J Parasitol 2008;94(2):467-472
https://doi.org/10.1645/GE-1365.1
8. Lee IS, Lee EJ, Park JB, Baek SH, Oh CS, et al. Acute traumatic death of a 17th century general based on examination of mummified remains found in Korea. Ann Anat 2009;191(3):309-320
https://doi.org/10.1016/j.aanat.2009.02.006
9. Seo M, Hong JH, Reinhard K, Shin DH. Archaeoparasitology of Korean mummies. In Shin DH, Bianucci R eds, The Handbook of Mummy Studies; Springer Nature; Singapore: 2021. 439-450
https://doi.org/10.1007/978-981-15-3354-9_14
10. Oh CS, Seo M, Lim NJ, Lee SJ, Lee EJ, et al. Paleoparasitological report on Ascaris aDNA from an ancient east Asian sample. Mem Inst Oswaldo Cruz 2010;105(2):225-228
https://doi.org/10.1590/S0074-02762010000200020
11. Oh CS, Seo M, Chai JY, Lee SJ, Kim MJ, et al. Amplification and sequencing of Trichuris trichiura ancient DNA extracted from archaeological sediments. J Archaeol Sci 2010;37(6):1269-1273
https://doi.org/10.1016/j.jas.2009.12.029
12. Shin DH, Oh CS, Lee HJ, Chai JY, Lee SJ, et al. Ancient DNA analysis on Clonorchis sinensis eggs remained in samples from medieval Korean mummy. J Archaeol Sci 2013;40(1):211-216
https://doi.org/10.1016/j.jas.2012.08.009
13. Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. 9th ed. Lea & Fibiger. Philadelphia, USA. 1984.
14. Shin DH, Shim SY, Kim MJ, Oh CS, Lee MH, et al. V-shaped pits in regions of ancient Baekje Kingdom paleoparasitologically confirmed as likely human-waste reservoirs. Korean J Parasitol 2014;52(5):569-573
https://doi.org/10.3347/kjp.2014.52.5.569
15. Oh CS, Seo M, Hong JH, Chai JY, Oh SW, et al. Ancient mitochondrial DNA analyses of Ascaris eggs discovered in coprolites from Joseon tomb. Korean J Parasitol 2015;53(2):237-242
https://doi.org/10.3347/kjp.2015.53.2.237
16. Hong JH, Oh CS, Seo M, Chai JY, Shin DH. Ancient Ascaris DNA sequences of cytochrome B, cytochrome C oxidase subunit 1, NADH dehydrogenase subunit 1, and internal transcriber spacer 1 genes from Korean Joseon mummy feces. J Parasitol 2017;103(6):795-800
https://doi.org/10.1645/16-102
17. Leles D, Gardner SL, Reinhard K, Iñiguez A, Araújo A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasit Vectors 2012;5:42
https://doi.org/10.1186/1756-3305-5-42
18. Shin DH, Chai JY, Park EA, Lee W, Lee H, et al. Finding ancient parasite larvae in a sample from a male living in late 17th century Korea. J Parasitol 2009;95(3):768-771
https://doi.org/10.1645/GE-1763.1
19. Shin DH, Oh CS, Chung T, Yi YS, Chai JY, et al. Detection of parasite eggs from a moat encircling the royal palace of Silla, the ancient Korean Kingdom. J Archaeol Sci 2009;36:2534-2539
https://doi.org/10.1016/j.jas.2009.07.009
20. Shin DH, Lim DS, Choi KJ, Oh CS, Kim MJ, et al. Scanning electron microscope study of ancient parasite eggs recovered from Korean mummies of the Joseon Dynasty. J Parasitol 2009;95(11):137-145
https://doi.org/10.1645/GE-1588.1
21. Seo M, Oh CS, Chai JY, Lee SJ, Park JB, et al. The influence of differential burial preservation on the recovery of parasite eggs in soil samples from Korean medieval tombs. J Parasitol 2010;96(2):366-370
https://doi.org/10.1645/GE-2131.1
22. Shin DH, Oh CS, Lee SJ, Chai JY, Kim J, et al. Paleo-parasitological study on the soils collected from archaeological sites in old districts of Seoul City. J Archaeol Sci 2011;38(12):3555-3559
https://doi.org/10.1016/j.jas.2011.08.024
23. Shin DH, Oh CS, Chai JY, Lee HJ, Seo M.
Enterobius vermicularis eggs discovered in coprolites from a medieval Korean mummy. Korean J Parasitol 2011;49(3):323-326
https://doi.org/10.3347/kjp.2011.49.3.323
24. Shin DH, Oh CS, Shin YM, Cho CW, Ki HC, et al. The pattern of ancient parasite egg contamination in the private residence, alley, ditch and streambed soils of old Seoul City, the Capital of Joseon Dynasty. Int J Paleopathol 2013;3(3):208-213
https://doi.org/10.1016/j.ijpp.2013.04.002
25. Kim MJ, Shin DH, Song MJ, Song HY, Seo M. Paleoparasitological surveys for detection of helminth eggs in archaeological sites of Jeolla-do and Jeju-do. Korean J Parasitol 2013;51(4):489-492
https://doi.org/10.3347/kjp.2013.51.4.489
26. Seo M, Oh CS, Chai JY, Jeong MS, Hong SW, et al. The changing pattern of parasitic infection among Korean populations by paleoparasitological study of Joseon Dynasty mummies. J Parasitol 2014;100(1):147-150
https://doi.org/10.1645/12-60.1
27. Shin DH, Shim SY, Jeong HJ, Kim MJ, Lee MH, et al. A paleoparasitological study on the capital area of the ancient Korean Kingdom. J Parasitol 2015;101(4):458-461
https://doi.org/10.1645/14-515.1
28. Kim MJ, Seo M, Oh CS, Chai JY, Lee JJ, et al. Paleoparasitological study on the soil sediment samples from archaeological sites of Silla Kingdom in Korean peninsula. Quat Int 2016;405(part B):80-86
https://doi.org/10.1016/j.quaint.2015.02.007
29. Seo M, Oh CS, Hong JH, Chai JY, Cha SC, et al. Estimation of parasite infection prevalence of Joseon people by paleoparasitological data updates from the ancient feces of pre-modern Korean mummies. Anthropol Sci 2017;125(1):9-14
https://doi.org/10.1537/ase.160920
30. Cho PY, Park JM, Hwang MK, Park SH, Park YK, et al. Discovery of parasite eggs in archaeological residence during the 15th century in Seoul, Korea. Korean J Parasitol 2017;55(3):357-361
https://doi.org/10.3347/kjp.2017.55.3.357
31. Seo M, Oh CS, Hong JH, Chai JY, Ju JO, et al. Ancient soil-transmitted parasite eggs detected from the sixth century three kingdom period Silla tomb. J Korean Med Sci 2018;33:e53
https://doi.org/10.3346/jkms.2018.33.e53
32. Seo M, Shim SY, Lee HY, Kim Y, Hong JH, et al. Ancient echinostome eggs discovered in archaeological strata specimens from a Baekje capital ruins of South Korea. J Parasitol 2020;106(1):184-187
https://doi.org/10.1645/19-55
33. Oh CS, Lee H, Kim J, Hong JH, Cha SC, et al. Two helminthic cases of human mummy remains from Joseon-period graves in Korea. Korean J Parasitol 2021;59(2):149-152
https://doi.org/10.3347/kjp.2021.59.2.149
34. Oh CS, Shim SY, Kim Y, Hong JH, Chai JY, et al. Helminth eggs detected in soil samples of a possible toilet structure found at the capital area of ancient Baekje Kingdom of Korea. Korean J Parasitol 2021;59(4):393-397
https://doi.org/10.3347/kjp.2021.59.4.393
35. Oh CS, Chai JY, Min S, Oh KT, Seol J, et al. Updates on parasite infection prevalence in the Joseon period based on parasitological studies of human coprolites isolated from archaeological sites in the cities of Euijeongbu, Gumi, and Wonju. Parasites Hosts Dis 2023;61(1):89-93
https://doi.org/10.3347/PHD.22129
36. Leles D, Araújo A, Ferreira LF, Vicente ACP, Iñiguez AM. Molecular paleoparasitological diagnosis of Ascaris sp. from coprolites: new scenery of ascariasis in pre-Columbian South American times. Mem Inst Oswaldo Cruz 2008;103(1):106-108
https://doi.org/10.1590/S0074-02762008005000004
37. Loreille O, Roumat E, Verneau O, Bouchet F, Hänni C. Ancient DNA from Ascaris: extraction amplification and sequences from eggs collected in coprolites. Int J Parasitol 2001;31(10):1101-1106
https://doi.org/10.1016/S0020-7519(01)00214-4
38. Araújo A, Reinhard K, Ferreira LF, Pucu E, Chieffi PP. Paleoparasitology: the origin of human parasites. Arq Neuropsiquiatr 2013;71(9B):722-726
https://doi.org/10.1590/0004-282X20130159
39. Gonçalves MLC, Araújo A, Ferreira LF. Human intestinal parasites in the past: new findings and a review. Mem Inst Oswaldo Cruz 2003;98(suppl):103-118
https://doi.org/10.1590/S0074-02762003000900016
40. Shin DH, Oh CS, Chai JY, Ji MJ, Lee HJ, et al. Sixteen century Gymnophalloides seoi infection on the coast of the Korean Peninsula. J Parasitol 2012;98(6):1283-1286
https://doi.org/10.1645/GE-2920.1
41. Hong JH, Seo M, Oh CS, Shin DH. Genetic analysis of small-subunit ribosomal RNA, internal transcribed spacer 2, and ATP synthase subunit 8 of Trichuris trichiura ancient DNA retrieved from the 15th to 17th century Joseon Dynasty mummies. J Parasitol 2019;105(4):539-545
https://doi.org/10.1645/19-31
42. Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 6th ed. The English Language Book Society. Baillière, Tindall & Cassell Ltd. London, UK. 1968.
43. Feliu C, Spakulová M, Casanova JC, Renaud F, Morand S, et al. Genetic and morphological heterogeneity in small rodent whipworms in Southwestern Europe: characterization of Trichuris muris and description of Trichuris arvicolae n. sp. (Nematoda: Trichuridae). J Parasitol 2000;86(3):442-449
https://doi.org/10.1645/0022-3395(2000)086[0442:GAMHIS]2.0.CO;2
44. Yoshikawa H, Yamada M, Matsumoto Y, Yoshida Y. Variations in egg size of Trichuris trichiura.
. Parasitol Res 1989;75(8):649-654
https://doi.org/10.1007/BF00930964
45. Nejsum P, Andersen KL, Andersen SD, Thamsborg SM, Tejedor AM. Mebendazole treatment persistently alters the size profile and morphology of Trichuris trichiura eggs. Acta Trop 2020;204:105347
https://doi.org/10.1016/j.actatropica.2020.105347
46. Ryoo S, Jung BK, Hong S, Shin H, Song H, et al. Standard- and large-sized eggs of Trichuris trichiura in the feces of schoolchildren in the Yangon Region, Myanmar: morphological and molecular analyses. Parasites Hosts Dis 2023;61(3):317-324
https://doi.org/10.3347/PHD.23059
47. Flammer PG, Dellicour S, Preston SG, Rieger D, Warren S, et al. Molecular archaeoparasitology identifies cultural changes in the Medieval Hanseatic trading centre of Lübeck. Proc R Soc B 2018;285(1888):20180991
https://doi.org/10.1098/rspb.2018.0991
48. Ferreira LF, Reinhard KJ, Araújo A. The origin of parasites of humans. In Ferreira LF, Reinhard KJ, Araújo A eds, Foundations of Paleoparasitology. Oswaldo Cruz Foundation. Rio de Janeiro, Brazil. 2014, pp 121-139.
49. Reinhard KL, Hevly RH, Anderson GA. Helminth remains from prehistoric Indian coprolites on the Colorado Plateau. J Parasitol 1987;73(3):630-639
https://doi.org/10.2307/3282147
50. Reinhard KJ, Ambler JR, McGuffie M. Diet and parasitism at Dust Devil Cave. Am Antiq 1985;50(4):819-824
https://doi.org/10.2307/280170
51. Manter HW. Some aspects of the geographical distribution of parasites. J Parasitol 1967;53(1):1-9
https://doi.org/10.2307/3276610
52. Moravec F. Redescription and systematic status of Capillaria philippinensis, an intestinal parasite of human beings. J Parasitol 2001;87(1):161-164
https://doi.org/10.1645/0022-3395(2001)087[0161:RASSOC]2.0.CO;2
53. Borba VH, Machado-Silva JR, Le Bailly M, Iñiguez M. Worldwide paleodistribution of capillarid parasites: paleoparasitology, current status of phylogeny and taxonomic perspectives. PLoS One 2019;14(4):e0216150
https://doi.org/10.1371/journal.pone.0216150
54. Choe G, Lee HS, Seo JK, Chai JY, Lee SH, et al. Hepatic capillariasis: first case report in the Republic of Korea. Am J Trop Med Hyg 1993;48(5):610-625
https://doi.org/10.4269/ajtmh.1993.48.610
55. Lee SH, Hong ST, Chai JY, Kim WH, Kim YT, et al. A case of intestinal capillariasis in the Republic of Korea. Am J Trop Med Hyg 1993;48(4):542-546
https://doi.org/10.4269/ajtmh.1993.48.542
56. Jung WT, Kim HJ, Min HJ, Ha CY, Kim HJ, et al. An indigenous case of intestinal capillariasis with protein-losing enteropathy in Korea. Korean J Parasitol 2012;50(4):333-337
https://doi.org/10.3347/kjp.2012.50.4.333
57. Pike AW. Recovery of helminth eggs from archaeological excavations, and their possible usefulness in providing evidence for the purpose of an occupation. Nature 1968;219(5151):303-304
https://doi.org/10.1038/219303a0
58. Bouchet F. Intestinal capillariasis in neolithic inhabitants of Chalain (Jura, France). Lancet 1997;349(9047):256
https://doi.org/10.1016/S0140-6736(05)64868-4
59. Valverde G, Ali V, Duran P, Castedo L, Paz JL. First report in pre-Columbian mummies from Bolivia of Enterobius vermicularis infection and capillarid eggs: a contribution to paleoparasitology studies. Int J Paleopathol 2020;31:34-37
https://doi.org/10.1016/j.ijpp.2020/08.002
60. Wei O. Internal organs of a 2100-year-old female corpse. Lancet 1973;102(7839):1198
https://doi.org/10.1016/S0140-6736(73)92953-X
61. Horne PD. First evidence of enterobiasis in ancient Egypt. J Parasitol 2002;88(5):1019-1021
https://doi.org/10.2307/3285550
62. Nazamabadi M, Aali A, Stöllner Th, Mashkour M, Bailley M. Paleoparasitological analysis of samples from the Chehrabad salt mine (Northwest Iran). Int J Paleopathol 2013;3(3):229-233
https://doi.org/10.1016/j.ijpp.2013.03.003
63. Fry GF, Moore JG.
Enterobius vermicularis: 10,000-year-old human infection. Science 1969;166(3913):1620
https://doi.org/10.1126/science.166.3913.1620
64. Kiliks MM. Helminths as heirlooms and souvenirs: a review of New World paleoparasitology. Parasitol Today 1990;9(4):93-100
https://doi.org/10.1016/0169-4758(90)90223-Q
65. Araújo A, Reinhard K, Ferreira LF. Paleoparasitology – human parasites in ancient material. Adv Parasitol 2015;90:349-387
https://doi.org/10.1016/bs.apar.2015.03.003
66. Hugot JP, Gardner SL, Borba V, Araujo P, Leles D, et al. Discovery of a 240 million year old nematode parasite egg in a cynodont coprolite sheds light on the early origin of pinworms in vertebrates. Parasit Vectors 2014;7:486
https://doi.org/10.1186/s13071-014-0486-6
67. Harter-Lailheugue S, Mort FD, Vigne JD, Guilaine J, Brun AL, et al. Premiéres Données Parasitologiques sur les populations humaines précéramiques chypriotes (VIIe et VIIe millénaires av. J.-C.). Paléorient 2005;31(2):43-54 (in French). https://doi.org/10.3406/paleo.2005.5124
68. Dommelier S, Bentrad S, Paicheler JC, Pétrequin P, Bouchet F. Parasitoses liées à L’alimentation chez les populations Néolithiques du Lac de Chalain (Jura, France). Anthropozoologica 1998;27:41-49 (in French).
69. Maicher C, Bleicher N, Le Bailly M. Spatializing data in paleoparasitology: application to the study of the neolithic lakeside settlement of Zürich-Parkhaus-Opéra, Switzerland. The Holocene 2019;29(7):1198-1205
https://doi.org/10.1177/0959683619838046
70. David AR. Disease in Egyptian mummies: the contribution of new technologies. Lancet 1997;349(9067):1760-1763
https://doi.org/10.1016/S0140-6736(96)10221-X
71. Vásquez AA, Alba A, Alda P, Vittecoq M, Hurtrez-Boussès S. On the arrival of fasciolosis in the Americas. Trends Parasitol 2022;38(3):195-204
https://doi.org/10.1016/j.pt.2021.12.001
72. Mas-Coma S, Valero MA, Bargues MD. Human and animal fascioliasis: origins and worldwide evolving scenario. Clin Microbiol Rev 2022;35(4):e0008819
https://doi.org/10.1128/cmr.00088-19
73. Le Bailly S, Bouchet F. Ancient dicrocoeliasis: occurrence, distribution and migration. Acta Trop 2010;115(3):175-180
https://doi.org/10.1016/j.actatropica.2010.03.004
74. Miyazaki I. Helminthic Zoonoses. International Medical Foundation of Japan. Tokyo, Japan. 1991.
75. Jouy-Avantin F, Combes C, Lumley H, Miskovsky JC, Moné H. Helminth eggs in animal coprolites from a Middle Pleistocene site in Europe. J Parasitol 1999;85(2):376-379
https://doi.org/10.2307/3285652
76. Knorr DA, Smith WPW, Ledger ML, Peña-Chocarro L, Pérez-Jordà G, et al. Intestinal parasites in six Islamic medieval period latrines from 10th~11th century Córdoba (Spain) and 12th~13th century Mértola (Portugal). Int J Paleopathol 2019;26:75-83
https://doi.org/10.1016/j.ijpp.2019.06.004
77. Slepchenko S, Reinhard K. Paleoparasitology and pathoecology in Russia: investigations and perspectives. Int J Paleopathol 2018;22:39-44
https://doi.org/10.1016/j.ijpp.2018.03.005
78. Makki M, Dupouy-Camet J, Sajaadi SMS, Naddaf SR, Mobedi I, et al. First paleoparasitological report on the animal feces of bronze age excavated from Shahr-e Sukhteh, Iran. Korean J Parasitol 2017;55(2):197-201
https://doi.org/10.3347/kjp.2017.55.2.197
79. Yeh HY, Cheng CJ, Huang CJ, Zhan X, Wong WK, et al. Discovery of Eurytrema eggs in sediment from a colonial period latrine in Taiwan. Korean J Parasitol 2019;57(6):595-599
https://doi.org/10.3347/kjp.2019.57.6.595
80. Chai JY, Jung BK. General overview of the current status of human foodborne trematodiasis. Parasitology 2022;149(10):1262-1285
https://doi.org/10.1017/S0031182022000725
81. Hong JH, Oh CS, Chai JY, Seo M, Shin DH. Cytochrome c oxidase subunit 1, internal transcribed spacer 1, nicotinamide adenine dinucleotide hydrogen dehydrogenase subunits 2 and 5 of Clonorchis sinensis ancient DNA retrieved from Joseon Dynasty mummy specimens. J Korean Med Sci 2019;34(20):e149
https://doi.org/10.3346/jkms.2019.34.e149
82. Oh CS, Seo M, Lee HJ, Kim MJ, Lim DS, et al. Genetic analysis of ancient Clonorchis sinensis eggs attained from Goryeong mummy of Joseon Dynasty period. J Parasitol 2022;108(1):70-78
https://doi.org/10.1645/21-49
83. Chai JY. Human intestinal flukes-From Discovery to Treatment and Control. Springer Nature B. V. Dordrecht. The Netherlands. 2019, pp 1-549.
84. Lee SH, Hwang SW, Chai JY, Seo BS. Comparative morphology of eggs of heterophyids and Clonorchis sinensis causing human infections in Korea. Korean J Parasitol 1984;22(2):171-180
https://doi.org/10.3347/kjp.1984.22.2.171
85. Chai JY, Jung BK. Epidemiology of trematode infections: an update. Adv Exp Med Biol 2019;1154:359-409
https://doi.org/10.1007/978-3-030-18616-6_12
86. Wang T, Mitchell PD. Liver fluke infection throughout human evolution. Gastro Hep Adv 2022;1(4):500-507
https://doi.org/10.1016/j.gastha.2022.02.027
87. Su TC. A scanning electron microscopic study on the parasite eggs in an ancient corpse from a tomb of Chu Dynasty, the Warring State, in Jiangling County, Hubei province. J Tongji Med Univ 1987;7(1):83-84
https://doi.org/10.1007/BF02888148
88. Yeh HY, Mitchell PD. Ancient human parasites in ethnic Chinese populations. Korean J Parasitol 2016;54(5):565-572
https://doi.org/10.3347/kjp.2016.54.5.565
89. Zhan X, Qi W, Yeh HY. Ancient parasites seen in the archaeology and medical contexts in the Han Dynasty, China. In Shin DH, Bianucci R eds, The Handbook of Mummy Studies. Springer. Singapore. 2021, pp 513-532
https://doi.org/10.1007/978-981-15-3354-9_41
90. Matsui A, Kanehara M, Kanehara M. Paleoparasitology in Japan--Discovery of toilet features. Mem Inst Oswaldo Cruz 2003;98(suppl):127-136
https://doi.org/10.1590/S0074-02762003000900019
91. Reinhard KJ, Araújo A, Sianto L, Costello JG, Swope K. Chinese liver flukes in latrine sediments from Wong Nim’s property, San Bernadino, California: archaeoparasitology of the Caltrans District Headquarters. J Parasitol 2008;94(1):300-303
https://doi.org/10.1645/GE-1049.1
92. Kubiak-Martens L, van der Linden M, Hardy K, Mackay H, Ngan-Tillard DJM, et al. Synthesis-human versus community diet. In Kubiak-Martens L, van der Linden M eds, Neolithic Human Diet Based on Studies of Coprolites from the Swifterbant Culture Sites, the Netherlands. Cultural Heritage Agency of the Netherlands. of the Netherlands. 2022, pp 119-139.
93. Anastasiou E. Parasites in European populations from prehistory to the industrial revolution. In Mitchell PD ed, Sanitation, Latrines and Intestinal Parasites in Past Populations. Routledge, Taylor and Francis. London & New York, UK. 2015, pp 203-217.
94. Moore JG, Grundmann AW, Hall HJ, Fry GF. Human fluke infection in Glen Canyon at AD 1250. Am J Phyiol Anthropol 1974;41(1):115-118
https://doi.org/10.1002/ajpa.1330410115
95. Horne PD. A review of the evidence of human endoparasitism in the pre-Columbian New World through the study of coprolites. J Archaeol Sci 1985;12(4):299-310
https://doi.org/10.1016/0305-4403(85)90035-4
96. Slepchenko SM, Gusev AV, Ivanov SN, Svyatova O. Opisthorchiasis in infant remains from the medieval Zeleniy Yar burial ground of XII-XIII centuries AD. Mem Inst Oswaldo Cruz 2015;110(8):974-980
https://doi.org/10.1590/0074-02760150156
97. Slepchenko SM, Ivanov SN, Gusev AV, Svyatova O, Fedorova NV. Archaeological and palynological snalysis of samples from the intestinal contents of a child mummy from the Zeleniy Yar burial ground (12~13th centuries AD). Archaeol Res Asia 2019;17:133-136
https://doi.org/10.1016/j.ara.2018.10.005
98. Slepchenko SM, Kardash OV, Slavinsky VS, Ivanov SN, Sergeyevna RD, et al. Archaeological analysis of samples from the cultural layer of Nadym Gorodok dated back to the 14th-late 18th centuries. Korean J Parasitol 2019;57(6):567-573
https://doi.org/10.3347/kjp.2019.57.6.567
99. Slepchonko S, Seo M, Hong JH, Oh CS, Shin DH. Mummies and skeletonized individuals to reveal the relationship of parasitism, social complexity, and subsistence strategy in Eurasian Continent. In Shin DH, Bianucci R eds, The Handbook of Mummy Studies; Springer Nature; Singapore: 2021. 1-15
https://doi.org/10.1007/978-981-15-1614-6_45-2
100. Steverding D. The spreading of parasites by human migratory activities. Virulence 2020;11(1):1177-1191
https://doi.org/10.1080/21505594.2020.1809963
101. Shin DH, Oh CS, Lee SJ, Lee EJ, Yim SG, et al. Ectopic paragonimiasis from 400-year-old female mummy of Korea. J Archaeol Sci 2012;39(4):1103-1110
https://doi.org/10.1016/j.jas.2011.12.011
102. Shin DH, Kim YS, Yoo DS, Kim MJ, Oh CS, et al. A case of ectopic paragonimiasis in a 17th century Korean mummy. J Parasitol 2017;103(4):399-403
https://doi.org/10.1645/16-63
103. Hong JH, Oh CS, Seo M, Shin DH. Analysis of COI and ITS2 regions of DNA obtained from Paragonimus westermani eggs in ancient coprolites on Joseon Dynasty mummies. Mem Inst Oswaldo Cruz 2019;114:e180595
https://doi.org/10.1590/0074-02760180595
104. Mitchell PD. The origin of human parasites: exploring the evidence for endoparasitism throughout human evolution. Int J Paleopathol 2013;3(3):191-198
https://doi.org/10.1016/j.ijpp.2013.08.003
105. Hernández-Chea R, Jiménez-Rocha AE, Castro R, Blair D, Dolz G. Morphological and molecular characterization of the metacercaria of Paragonimus caliensis, as a separate species from P. mexicanus in Costa Rica. Parasitol Int 2017;66(2):126-133
https://doi.org/10.1016/j.parint.2016.12.006
106. Hong JH, Seo M, Oh CS, Chai JY, Shin DH.
Metagonimus yokogawai ancient DNA recovered from 16th- to 17th-century Korean mummy feces of the Joseon Dynasty. J Parasitol 2020;106(6):802-808
https://doi.org/10.1645/20-42
107. Oh CS, Hong JH, Chai JY, Song MK, Jang HJ, et al. Ancient DNA of Metagonimus yokogawai recovered from Joseon period human remains newly discovered at Goryeong county in South Korea. Acta Parasitol 2022;67(1):539-545
https://doi.org/10.1007/s11686-021-00487-0
108. Kim CH. Study on the Metagonimus sp. in Gum River basin, Chungchung-nam do, Korea. Korean J Parasitol 1980;18(2):215-228 (in Korean). https://doi.org/10.3347/kjp.1980.18.2.215
109. Kim CH, Kim NM, Lee CH, Park JS. Studies on the Metagonimus fluke in the Daecheong Reservoir and the upper stream of Geum River, Korea. Korean J Parasitol 1987;25(1):69-82
https://doi.org/10.3347/kjp.1987.25.1.69
110. Thaenkham U, Blair D, Nawa Y, Waikagul J. Families Opisthorchiidae and Heterophyidae: are they distinct? Parasitol Int 2012;61(1):90-93
https://doi.org/10.1016/j.parint.2011.06.004
111. Seo M, Chai JY, Kim MJ, Shim SY, Ki HC, et al. Detection trend of helminth eggs in the strata soil samples from ancient historic places of Korea. Korean J Parasitol 2016;54(5):555-563
https://doi.org/10.3347/kjp.2016.54.5.555
112. Zimmerman MR, Smith GS. A probable case of accidental inhumation of 1,600 years ago. Bull N Y Acad Med 1975;51(7):828-837.
113. Chai JY, Choi MH, Yu JR, Lee SH.
Gymnophalloides seoi: a new human intestinal trematode. Trends Parasitol 2003;19(3):109-112
https://doi.org/10.1016/S1471-4922(02)00068-5
114. Lee SH, Chai JY. A review of Gymnophalloides seoi (Digenea: Gymnophallidae) and human infections in the Republic of Korea. Korean J Parasitol 2001;39(2):85-118
https://doi.org/10.3347/kjp.2003.39.2.85
115. Sianto L, Reinhard KJ, Chame M, Chaves S, Mendonça SM, et al. The finding of Echinostoma (Trematoda: Digenea) and hookworm eggs in coprolites collected from a Brazilian mummified body dated 600–1,200 years before present. J Parasitol 2005;91(4):972-975
https://doi.org/10.1645/GE-3445RN.1
116. Leles D, Cascardo P, dos Santos Freire A, Maldonado A Jr, Sianto L, et al. Insights about echinostomiasis by paleomolecular diagnosis. Parasitol Int 2014;63(4):646-649
https://doi.org/10.1016/j.parint.2014.04.005
117. Jiménez FA, Gardner SL, Araújo A, Fugassa M, Brooks RH, et al. Zoonotic and human parasites of inhabitants of Cueva de los Muertos Chiquitos, Rio Zape Valley, Durango, Mexico. J Parasitol 2012;98(2):304-309
https://doi.org/10.1645/GE-2915.1
118. Rabinow S, Deforce K, Mitchell PD. Continuity in intestinal parasite infection in Aalst (Belgium) from the medieval to early modern period (12th–17th centuries). Int J Paleopathol 2013;41:43-49
https://doi.org/10.1016/j.ijpp.2023.03.001
119. Sianto L, Duarte AN, Borba VH, Magalhães JG, de Souza SM, et al. Echinostomes in felid coprolites from Brazil. J Parasitol 2016;102(3):385-387
https://doi.org/10.1645/15-819
120. Waeschenbach A, Brabec J, Scholz T, Littlewood DTJ, Kuchta R. The catholic taste of broad tapeworms-multiple routes to human infection. Int J Parasitol 2017;47:831-843
https://doi.org/10.1016/j.ijpara.2017.06.004
121. Le Bailly M, Bouchet F.
Diphyllobothrium in the past: review and new records. Int J Paleopathol 2013;3(3):182-187
https://doi.org/10.1016/j.ijpp.2013.05.004
122. Jeon HK, Kim KH, Huh S, Chai JY, Min DY, et al. Morphologic and genetic identification of Diphyllobothrium nihonkaiense in Korea. Korean J Parasitol 2009;47(4):369-375
https://doi.org/10.3347/kjp.2009.47.4.369
123. Choi S, Cho J, Jung BK, Kim DG, Jeon SJ, et al.
Diphyllobothrium nihonkaiense: wide egg size variation in 32 molecularly confirmed adult specimens from Korea. Parasitol Res 2015;114:2129-2134
https://doi.org/10.1007/s00436-015-4401-7
124. Sianto L, Chame M, Silva CSP, Gonçalves MLC, Reinhard K, et al. Animal helminths in human archaeological remains: a review of zoonoses in the past. Res Inst Med Trop S Paulo 2009;51(3):119-130
https://doi.org/10.1590/S0036-46652009000300001
125. Le Bailly M, Bouchet F.
Diphyllobothrium in the past: review and new records. Int J Paleopathol 2013;3(3):182-187
https://doi.org/10.1016/j.ijpp.2013.05.004
126. Gaeta R, Fornaciari G. Paleoparasitology of helminths. In Bruschi ed, Helminth Infections and Their Impact on Global Public Health; Springer Nature. Switzerland AG; Cham, Switzerland: 2022.
https://doi.org/10.1007/978-3-031-00303-5_3
127. Harter S. Implication de la Paléoparasitologie dans l’étude des populations anciennes de la vallée du Nil et de proche-orient: étude de cas. Médecine humaine et pathologie. Ph.D. thesis. Université de Reims-Champagne Ardenne; France: 2003. (in French).
128. Slepchenko SM, Lobanova TV, Zizgalov GP, Alyamkin GV, Ivanov SN. New contribution of archaeoparasitology in the Far North of Eastern Siberia: first data about the parasitological spectrum of Stadukhinsky Fort in the 17th–18th centuries. J Archaeol Sci: Reports 2022;41:103304
https://doi.org.10.1016/j.jasrep.2021.103304
129. Hoberg EP, Gardner SL, Campbell RA. Systematics of the Eucestoda: advances toward a new phylogenetic paradigm, and observations on the early diversification of tapeworms and vertebrates. Syst Parasitol 1999;42(1):1-12
https://doi.org/10.1023/A:1006099009495
130. Hoberg EP.
Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes Infect 2002;4(8):859-866
https://doi.org/10.1016/S1286-4579(02)01606-4
131. Lee HJ, Shin DH, Seo M. Discovery of taeniid eggs from a 17th century tomb in Korea. Korean J Parasitol 2011;49(3):327-329
https://doi.org/10.3347/kjp.2011.49.3.327
132. Reinhard KJ. Archaeoparasitology in North America. Am J Physic Anthropol 1990;82(2):145-163
https://doi.org/10.1002/ajpa.1330820204
133. Bailly Le, Evolution M. de la hôte/parasite dans les systèmes lacustres nord alpins au Néolithique 3900–2900 BC), et nouvelles données dans la détection des paléoantigènes de Protozoa
. Ph.D. thesis. Universite de Reims Champagne-Ardenne; France: 2005. (in French).
134. Hoberg EP, Alkire NL, de Quiroz A, Jones A. Out of Africa: origins of the Taenia tapeworms in humans. Proc R Soc 2001;268(1469):781-787
https://doi.org/10.1098/rspb.2000.1579
135. Fornaciari A, Gaeta R, Cavallini L, Aringhieri G, Ishak R. A 13th-century cystic echinococcosis from the cemetery of the monastery of Badia Pozzeveri (Lucca, Italy). Int J Paleopathol 2020;31:79-88
https://doi.org/10.1016/j/ijpp.2020.10.005
136. Ledger ML, Mitchell PD. Tracing zoonotic parasite infections throughout human evolution. Int J Osteoarchaeol 2019;32(3):553-564
https://doi.org/10.1002/oa.2786
137. Watson V, Rothschild B. Deep origin of parasitic disease in vertebrates. In Baets KD, Huntley JW eds, The Evolution and Fossil Record of Parasitism. Tropics in Geobiology book series. Springer. Cham, Switzerland. 2022.
138. Korea Association of Health Promoion. Prevalence of Intestinal Parasitic Infections in Korea—The 8th Report. Art Motion. Seoul, Korea. 2012.
139. Ki HC, Shin DH, Seo M, Chai JY. Infection patterns of trematode parasites among Joseon people. J Korean Med Assoc 2014;57(10):866-875 (in Korean). https://doi.org/10.5124/kma.2014.57.10.866
140. Shin DH, Blanucci R, Fujita H, Hong JH. Mummification in Korea and China: Mawangdui, Song, Ming and Joseon Dynasty mummies. BioMed Res Int 2018;2018:6215025
https://doi.org/10.1155/2018/6215025
141. Seo M, Chai JY, Hong JH, Shin DH. Reconsideration of Dr. Allen’s report about hemoptysis patients from high prevalence of archaeoparasitological paragonimiasis in Korea. Korean J Parasitol 2019;57(6):635-638
https://doi.org/10.3347/kjp.2019.57.6.635
142. Shin DH, Chai JY, Hong JH, Seo M. Historical details about the meat consumption and taeniases in Joseon period of Korea. Korean J Parasitol 2017;55(4):457-460
https://doi.org/10.3347/kjp.2016.55.4.457
Fig. 1Map showing the areas where archaeological remains examined were positive for helminth eggs and/or larvae in Korea (1997–2023). Black heptagons represent the areas where soil or strata soil specimens were examined (n=8), whereas white heptagons represent the areas where mummy samples (feces, tomb soil, or coprolites) were examined (n=32). Names of localities. 1, Gwangju; 2, Daegu; 3, Yangju; 4, Yongin; 5, Gyeongju-1; 6, Gongju; 7, Seocheon; 8–9, Seoul (Sinnae-dong A, B); 10, Seoul (old palace, etc.); 11, Dangjin; 12, Jangheung; 13, Paju; 14, Jinju; 15, Buyeo-1; 16, Buyeo-2; 17, Gimhae; 18, Changnyeong; 19, Yeounggwang-1; 20, Yeounggwang-2; 21, Dalsung; 22, Hwasung; 23, Seoul (Junggye); 24, Cheongdo; 25, Gyeongju-2; 26, Gwangmyeong; 27, Buyeo-3 (Hwajisan); 28, Wonju; 29, Gumi; 30, Gangneung; 31, Waegwan; 32–33, Seoul (Sinnae-dong C, D); 34, Sapgyo; 35, Mungyeong; 36, Andong; 37, Goryeong; 38, Euijeongbu-1; 39, Euijeongbu-2; 40, Hadong-1; 41, Hadong-2; 42, Sacheon. 
Fig. 2Sampling of archaeological specimens in paleoparasitological research in Korea. (A) Sampling sites (conical tubes) of strata soil in an old road of old Seoul City (near the Gyeongbok Palace). (B) A mummy from Cheongdo before examination in the laboratory. In this mummy, ectopic paragonimiasis was found in the liver tissue. 
Fig. 3
Ascaris lumbricoides eggs found in archaeological remains in Korea. (A) A fertilized egg detected from soil sediments in an old road of old Seoul City (near Gyeongbok Palace). It is almost round-shape containing a germ cell inside. It has a characteristic eggshell morphology, including the outer protein coat and inner chitin layer. Scale bar=25 μm. (B) An unfertilized egg (unpublished observation) detected from the coprolite of Cheongdo mummy. It is slightly elongated and elliptical in shape. It does not have a germ cell inside but is filled with undifferentiated granules. Scale bar=25 μm. 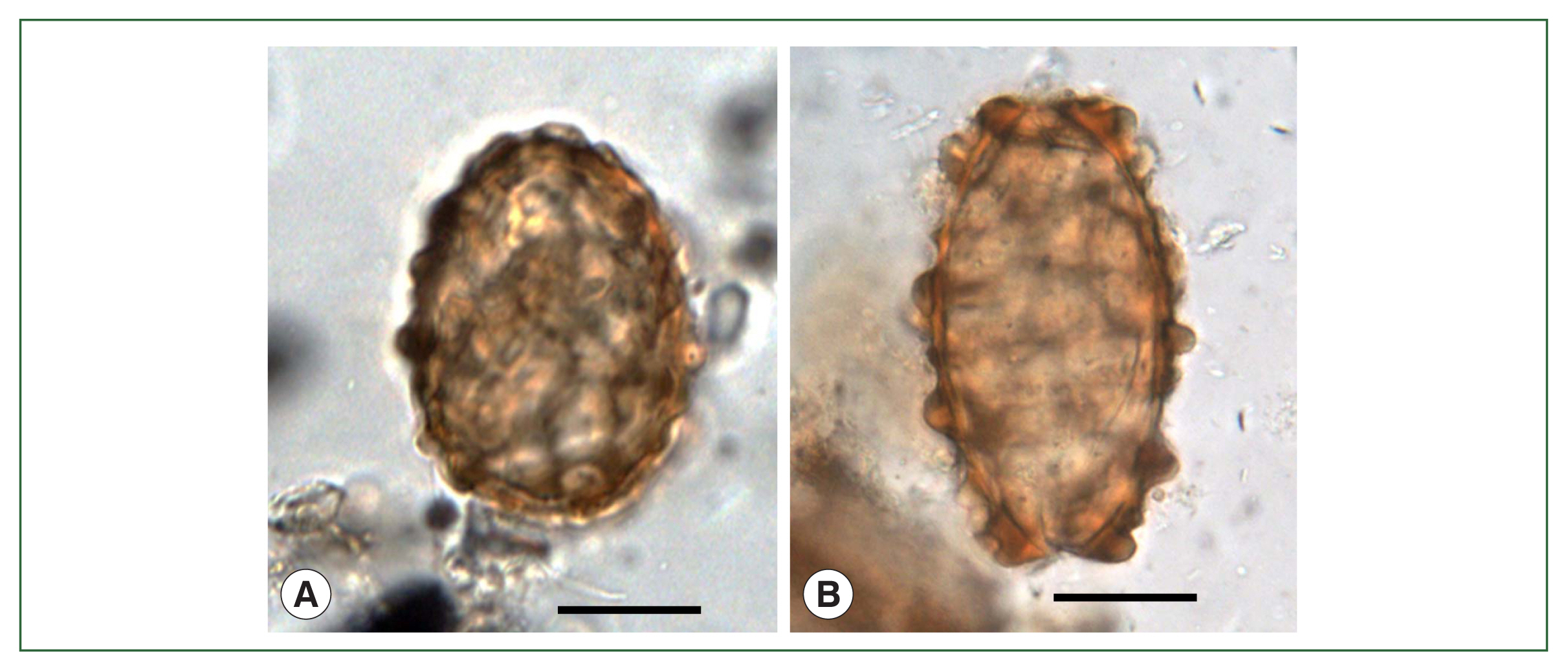
Fig. 4
Trichuris trichiura eggs found in archaeological samples in Korea. The eggs are barrel shaped with a thick eggshell and have prominent mucoid plugs on anterior and posterior ends. (A) An immature egg discovered in a strata soil specimen from an old road of old Seoul City (near the Gyeongbok Palace). Scale bar=25 μm. (B) A larva-containing mature egg found in the soil specimen (feces) of Sapgyo mummy. Scale bar=25 μm. 
Fig. 5
Clonorchis sinensis eggs detected in archaeological remains in Korea. They are ovoid or pyriform in shape with a prominent operculum, shoulder rims, and thick eggshell with a terminal knob. (A) An egg discovered in a V-shaped pit soil sample from Buyeo, an old capital of Baekje Dynasty. Scale bar=15 μm. (B) Another egg found in the tomb soil (feces) of Waegwan mummy. Scale bar= 15 μm. 
Fig. 6
Paragonimus westermani eggs found in archaeological specimens in Korea. (A) An egg discovered in the coprolite of Dangjin mummy. It has a prominent operculum, shoulder rims, and thick eggshell with abopercular thickening. Scale bar=20 μm. (B) Numerous eggs found in the soil sediment (probably feces) from the pelvic space of Hadong-2 mummy. Scale bar=200 μm. 
Fig. 7Upper abdominal view of Cheongdo mummy. In this mummy, ectopic paragonimiasis was confirmed in the liver tissue (arrow). Scale bar=2 cm. 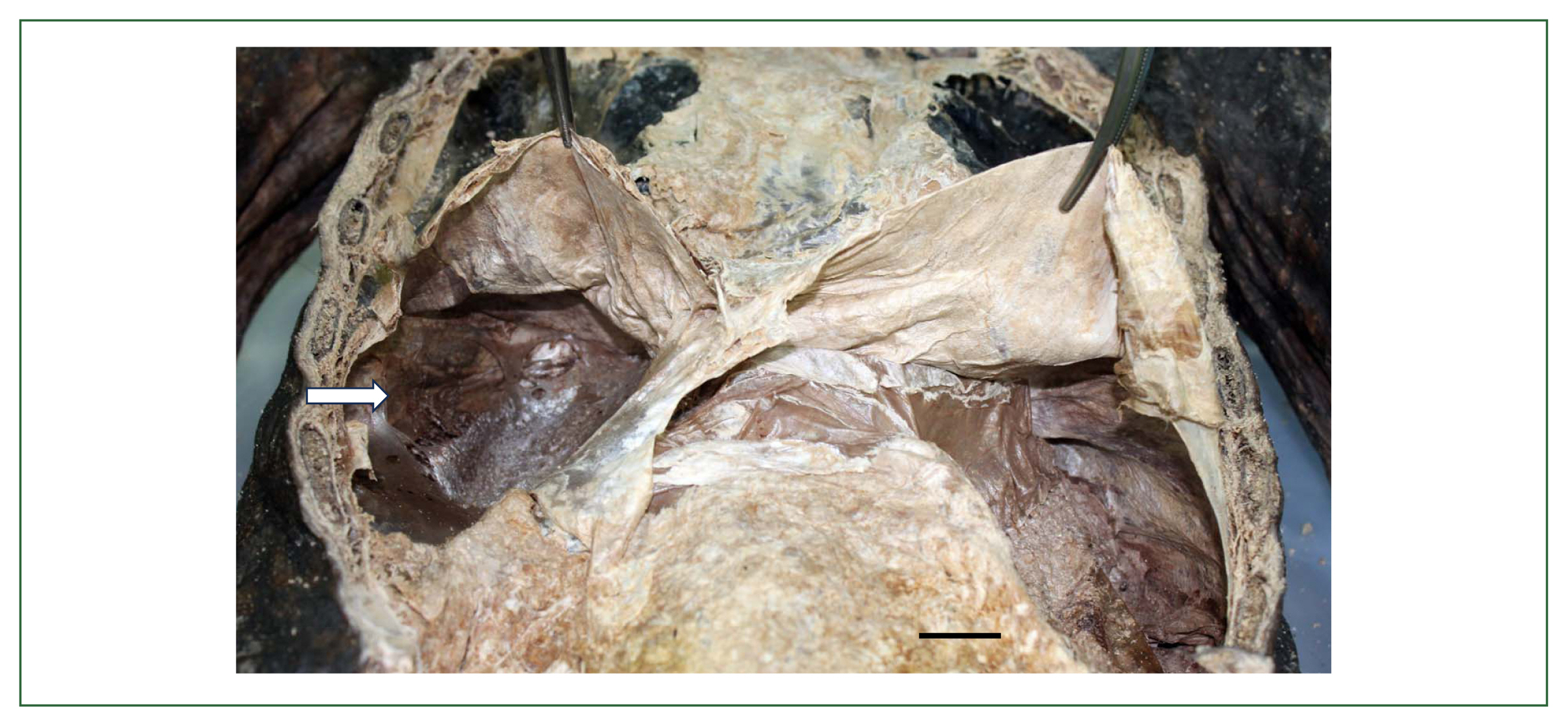
Fig. 8
Metagonimus yokogawai and Pygidiopsis summa eggs detected in archaeological remains in Korea. (A) M. yokogawai egg discovered in the coprolite of Sacheon mummy. It is almost elliptical shape, and the eggshell surface is clean with no shoulder rims. Scale bar=12 μm. (B) P. summa egg found in the strata soil from Buyeo (Hwajisan), the old capital of Baekje Dynasty. It is small and pyriform resembling C. sinensis egg. Scale bar=12 μm. 
Fig. 9
Gymnophalloides seoi eggs found in archaeological samples in Korea. (A) An egg discovered in the soil sediment (feces) of Sapgyo mummy. A large operculum is characteristically seen near the anterior end. Scale bar=20 μm. (B) High magnification of the egg in Fig. 9A. Scale bar=10 μm. 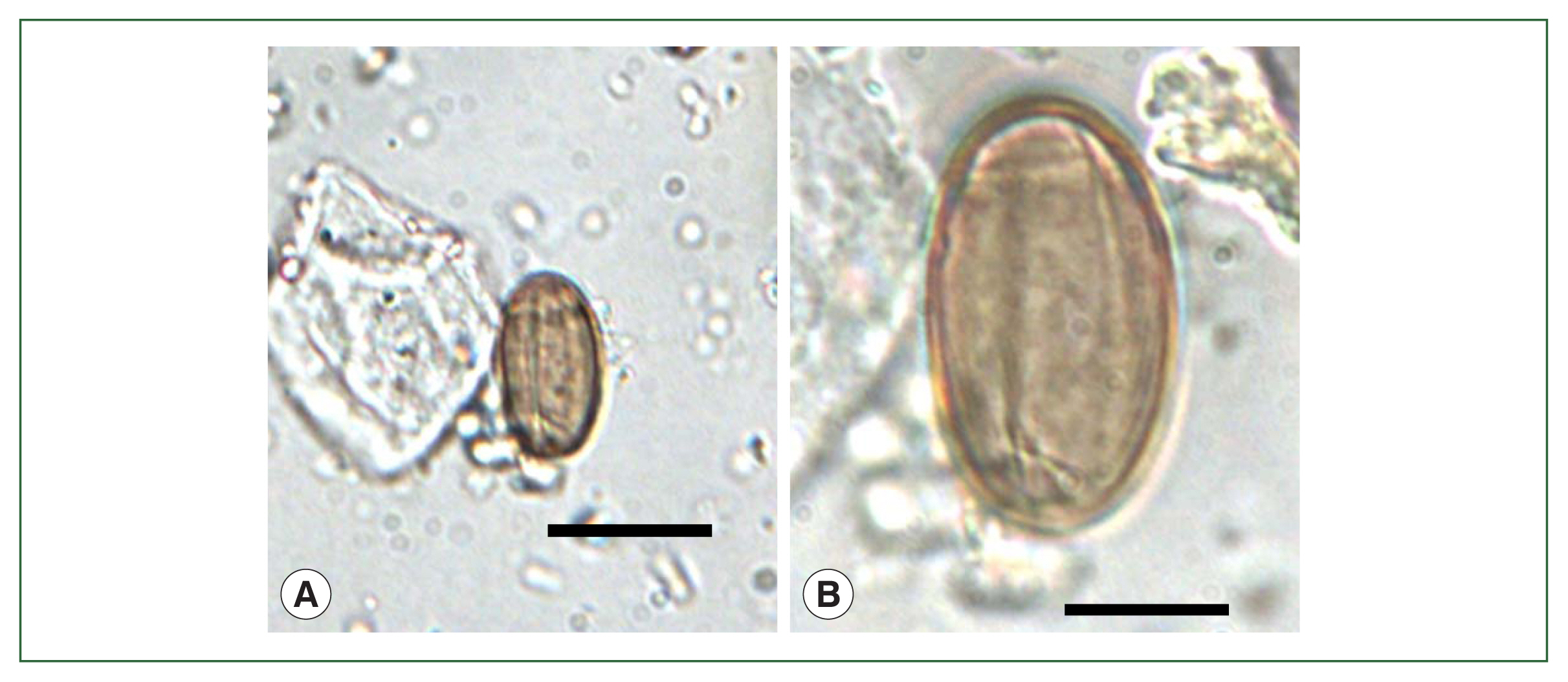
Fig. 10
Taenia spp. egg discovered in archaeological remains (A) in Korea in comparison with a typical contemporary egg (B) from Cambodia. (A) An egg found in the soil (feces) sample of Gongju mummy. Six hooklets inside the embryophore are difficult to recognize. Scale bar=25 μm. (B) A contemporary egg detected in a Kato-Katz fecal smear of a Cambodian resident (personal communication). Hooklets inside the egg are clearly visible. Scale bar=25 μm. 
Table 1Paleoparasitological reports on the detection of Ascaris lumbricoides or Ascaris sp. eggs in Korea
a Scanning electron microscopic study was done using the eggs obtained from the Yangju mummy [6]. b Molecular analyses were done using 18S ribosomal RNA (18S RNA) and mitochondrial cytochrome b (cyt b). Table 2Paleoparasitological reports on the detection of Trichuris trichiura or Trichuris sp. eggs in Korea
a Scanning electron microscopic study was done using the eggs obtained from the Yangju mummy [6]. Table 3Paleoparasitological reports on the detection of Strongyloides stercoralis and Trichostrongylus sp. larvae and Paracapillaria philippinensis (possibly) and Enterobius vermicularis eggs in Korea
Table 4Paleoparasitological reports on the detection of liver fluke eggs in Korea
c Molecular analysis was done using ITS1, cox1, NADH dehydrogenase subunit 2 and 5 (nd2 and nd5, respectively). d Molecular analysis was done using ITS1, ITS2, and cox1 genes on the eggs obtained from the Waegwan mummy [18]. Table 5Paleoparasitological reports on the detection of Paragonimus westermani eggs in Korea
a Scanning electron microscopic studies were done by Shin et al. in 2009 [20] using the eggs from this mummy. Table 6Paleoparasitological reports on the detection of intestinal fluke eggs in Korea
a M. yokogawai, Metagonimus yokogawai; P. summa, Pygidiopsis summa; G. seoi, Gymnophalloides seoi; I. hortensis, Isthmiophora hortensis. b Scanning electron microscopic studies were conducted by Shin et al. in 2009 [20] using the eggs from this mummy. c Molecular analysis was done by Hong et al. in 2020 [106] using 28S rDNA and mitochondrial cytochrome c oxidase subunit 1 (cox1). d Molecular analysis was done by Oh et al. in 2022 [107] using 28S rDNA and mitochondrial cytochrome c oxidase subunit 1 (cox1). Table 7Paleoparasitological reports on the detection of tapeworm eggs in archaeological specimens in Korea
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||