Human toxocariasis is a worlwide parasitic zoonosis caused by roundworm of dogs, Toxocara canis or roundworm of cats, Toxocara cati. Infection in human is caused by ingestion of embryonated Toxocara eggs present in soil, water, food, dirty hands, on vegetables or by ingestion of larvae from undercooked giblets. Through the digestion process in the host, larvae released from the eggs migrate with the blood through different tissues, causing inflammations. In adults, the larvae can cause weakness, pruritus, rash, difficult breathing and abdominal pain. This syndrome was named "common toxocariasis". Toxocara infection in children named also "covert toxocariasis" can cause fever, anorexia, headache, nausea, abdominal pain, lethargy, pharyngitis, pneumonia, wheezing, cough, cervical lymphadenitis, and hepatomegaly. Both mentioned syndromes can be with or without eosinophilia. The most frequent Toxocara infection in human can produce minor and self limiting syndromes so it is likely that many persons with these above-cited Toxocara clinical manifestations and laboratory abnormalities often go undiagnosed because of its non-specificity. The typical clinical syndromes of toxocariasis in human are visceral and ocular toxocariasis, named also visceral larva migrans (VLM) and ocular larva migrans (OLM). Visceral larva migrans syndrome including one or more of the following: eosinophilia associated with fever, general malaise, abdominal pain, headache, cough, anorexia, wheezing, hepatomegaly, anemia, leucocytosis, dermatologic manifestations as prurit or urticaria, pulmonary signs and neurological or cardiac syndromes. Ocular larva migrans can cause uveitis, posterior and peripheral retinochoroiditis, vitritis, endophthalmitis, papillitis and other ocular lesions that often lead to loss of vision in the affected eye. The most common affected sites by OLM are the peripheral retina and/or vitreous. When the inflammation resolves, a peripheral elevated white mass usually is seen, typically associated with retinal folds extending towards the macula. Eosinophilia is usually absent in ocular toxocariasis (Magnaval et al., 2001; Sabrosa and de Souza, 2001).
The aim of this study was to determine serologically the prevalence of Toxocara antibodies in patients, clinically suspected of ocular toxocariasis.
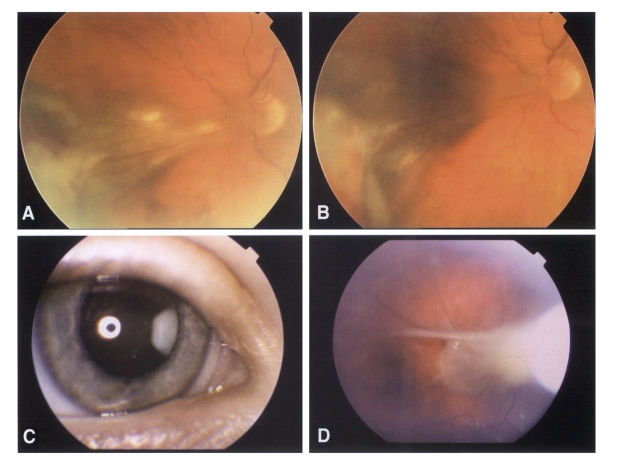
Between January 1, 2001 and the end of December 2003 at the Department of Parasitology, Institute of Microbiology and Immunology, Medical Faculty, Ljubljana, Slovenia, 239 serum samples of patients with the clinical manifestations of ocular inflammations, as posterior or peripheral retinochoroiditis, vitritis, papillitis or circumscript endophthalmitis were examined by ELISA T. canis IgG test (NOVATEC, Immundiagnostica GmbH) and confirmed by the Toxocara Western-blot IgG test (LDBIO DIAGNOSTICS, Lyon, France) (Fig. 1). ELISA sensitivity and specificity were 93.3% and 87.5% respectively; the cutoff value was calculated as mean optical density value (OD) of the two negative controls, plus 0.250. Optical density in the range of 10 % above to 10 % below the cut-off value was taken as grey zone. The presence on the Wetern blot strip of 2 or more low molecular weight bands in the range of 24-35 kDa was indicative for the presence of specific Toxocara IgG. All patients had affected one eye. The sera samples were obtained from patients of both sexes and varying ages, who came from different parts of Slovenia. They were not grouped by sex as no important differences in Toxocara seropositivity were found between males and females. There were no significant differences between patients from different regions either. Because toddlers and primary schoolchildren often come in contact with dirt and they frequently do not wash their hands before meals, which increases the risk of Toxocara infection in this population group, we devided 239 ocular patients sera in a group of 33 patients ranged three to 14 years and in group of 206 patients older than 14 years, aged to 82 years.
Seven sera of 239 patients with ELISA values of the grey zone and with negative Western blot test were considered Toxocara negative. None of the sera, positive by Western blot assay showed high molecular weight bands > 80 kDa which is less specific for Toxocara (it may cross-react with other helminthes). Of 239 patients suspected of ocular toxocariasis evaluated by serological tests, 171 (72%) were seronegative and 67 (28%) were Toxocara seropositive by both tests; 95% CI (22-34%). Toxocara positive sera ranged in ELISA OD values from 0.350-3.516. Out of 67 ELISA positive sera, 40 (59.7%) sera were low positive (0.350-1.000 OD values), 16 (23.9%) sera were positive (1.100-2.000 OD values) and 11 (16.4%) sera were high positive (2.100-3.516 OD values). The median age of 67 Toxocara seropositive patients was 37.6 years. The number of Toxocara seropositive ocular patients increased with age but there was no significant difference in the number of positive sera between the younger age group (6 of 33 samples) and the older age group (61 of 206 samples, χ2 = 1.84, p>0.05 (Table 1).
Toxocariasis was reported from countries throughout the world. It is a relatively new disease. The histological changes having been described by Wilder in 1950 and the causative organism was identified in 1956 by Nichols (Wilder, 1950; Nichols, 1956). It is generally accepted that the usual route of entry to the eye is via the blood stream and that a single larva in the eye may lead to potentially blinding condition (Samson et al., 2001; Taylor, 2001). Little is known about how soon after infection Toxocara antibodies start to rise and how long they remain elevated. A small number of larvae can supposedly escape the host immunity, if provoked at all, and reach the central nervous system, and possibly eye. This suggests that even low ELISA values may be indicative of ocular toxocariasis, but current immunodiagnostic tests are not capable of distinguishing between current infection, past infection or reactivation of "dormant larvae". It has been established that toxocariasis is caused mainly by T. canis but from the latest studies it is evident that also T. cati play a more important role than generally suggested. In an animal model (Mongolian gerbils) it was ascertained that T. cati larvae cause considerable eye lesions. Also results of micro-Ouchterlony test of patient's intraocular fluid led to the suggestion that T. cati might be more important as a cause of OLM than was previously belived. Sakai et al. (1998) found out in the macula lesion of the patient's eye, who lost visual acuity caused by a Toxocara spp., that the serum of this patient did not show strong reaction to excretoty/secretory (ES) antigen from T. canis larvae, but did show a strong reaction in ELISA with antigens of T. cati larvae (Petithory et al., 1993; Sakai et al., 1998; Fisher, 2003). By personal information of Western blot producer, their tests detect and confirm also infections caused by T. cati larvae, so it is suggested that some of our Toxocara positive patients could be infected by the eggs of T. cati. It was known that toxocariasis can be contracted through the ingestion of Toxocara eggs from contaminated soil or food but nowadays it is suggested that humans can be infected also by direct contact and than ingestion of emryonated Toxocara eggs in pet's hair (Wolfe and Wright, 2003).
Assuming that patients with ocular symptoms due to a suspected Toxocara infection in this study constitute a representative sample of these patients of Slovenia, the national prevalence of Toxocara antibodies in these patients, estimated on the basis of this representative sample is 22-34%, but it should be kept in mind that there may be some Toxocara seropositive cases without being associated with toxocariasis. Usually, also in our study it was diffucult to correlate between Toxocara positive serology results and severity of clinical manifestations. The mean age of Toxocara positive patients in the younger group was 9.5 years, but of the older group of patiens was 40 years. When we do not take into account two 70 years old patients and one 82 years old Toxocara positive patient of the older group, the mean age of this group was 38.4 years. These results accord with those who maintained that ocular larva migrans is unilateral and that mainly affects teenagers or young adults (Magnaval et al., 2001; Samson et al. 2001). We also found out that the incidence rate of Toxocara infections in ocular patients of Slovenia had increased from 21 to 28 % when compared two periods, 1990/91 and 2001/03 (Logar et al., 1993). It is suggested that the higher percent of Toxocara antibodies in ocular patients detected in this study might be the result of examination of clinically selected suspected cases of ocular toxocariasis, while in the first study patients with several ocular disorders were included. Another explanation for high percent of Toxocara antibodies in ocular patients found in this study might also be higher quality of diagnostic tests which are more specific and sensitive than 12 years ago when we used the ELISA test with Toxocara excretory/secretory (ES) antigen.
Likely in many other countries, also in Slovenia, Toxocara infection in humans can be ascribed to generally inadequate knowledge about the roundworms of dogs and cats, the route of infection and about preventive measures which include better personal hygiene and elimination of intestinal parasites from pets. It is suggested that of high prevalence of Toxocara seropositive cases in ocular patients, toxocariasis should be given more attention and that the ophthalmologists in Slovenia should be more aware of this disease — especially in children and young adults — and should more often include toxocariasis in the differential diagnosis of the ocular diseases.