INTRODUCTION
More than 10,000 cases of vivax malaria have occurred in the northern parts of Kyunggi-do and Kangwon-do provinces near the demilitarized zone (DMZ) of Korea (reviewed by Chai, 1999; Ree, 2000) after the first indigenous case of Plasmodium vivax infection was detected in 1993 (Chai et al., 1994). These regions are now regarded as endemic regions for vivax malaria. This re-emerging vivax malaria has been presumed to expand from the endemic regions north of DMZ because of changes in vector environments, although no information is available on the endemic status of the disease north of the DMZ.
In cases of vivax malaria, it is not possible to observe the parasite in blood smears during the irregularly prolonged incubation periods of vivax malaria in temperate climate regions (Krotoski, 1985). Incubation periods vary from 153 to 452 days before the onset of malarial symptoms in Korean cases (Lee et al., 1998). Though possible, it is not easy to examine especially when parasitemia is as low as it is in P. vivax infections. Various detection methods have been developed to overcome these limitations, such as antigen- (Dietze et al., 1995) and nucleic acid-based detection (Li et al., 1995) of falciparum malaria. Antibody-based detection methods such as the indirect haemagglutination test (WHO, 1988), the indirect fluorescent antibody test (Mendis et al., 1987), and ELISA tests (Demedts et al., 1987; Del Giudice et al., 1987) have been also established.
Previously we reported upon the availability of western blot for the serological diagnosis of vivax malaria, which had sensitivity comparable with those of the above-mentioned methods (Son et al., 2001). In the present study, we used ELISA and the stage-specific antigens chosen for vivax malaria to develop a method of serological diagnosis of vivax malaria. We then screened the sera of residents in endemic regions during the winter season when the symptoms are likely not to occur.
MATERIALS AND METHODS
Positive patients' sera and sera collected from residents in the endemic regions
Positive sera (152 cases) were selected by observing parasites in thin blood films stained with Diff-Quick solution (International Reagents Corp., Kobe, Japan) as described in Son et al. (2001). Serum was collected after centrifuging whole blood at 12,000 rpm and frozen at -70℃ until used. Negative healthy sera (128 cases) were collected from students in Seoul who had never visited the endemic areas.
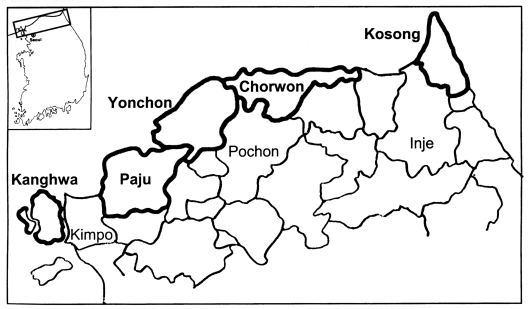
A total of 3,262 serum samples were collected from residents of the endemic regions from December 2000 to March 2001 in Kanghwa-gun, Paju-gun, and Yonchon-gun (Kyunggi-do) and in Chorwon-gun and Kosong-gun (Kangwon-do) (Fig. 1).
Production and purification of His-tagged antigens
DNA was extracted from the whole blood (200 µl) of a vivax malaria patient using a QIAamp DNA mini kit (Qiagen, Valencia, CA), according to the manufacturer's protocol. Primers were used as the same as described in Table 1 of Son et al. (2001). The denaturing and extension temperatures used for PCR were, 95℃ for 1 min and 72℃ for 2 min for 30 cycles.
Amplified DNA fragments of CSP-1, MSP-1, AMA-1, SERA, and EXP-1 were cloned into pGEM-T Easy vector (Promega, Madison, WI) and subcloned into pQE30 vector (Qiagen). Proteins were expressed individually as His-tagged forms in Escherichia coli (M15 strain) by isopropyl-β-D-thiogalactoside (IPTG) induction for 3 hr at 30℃. His-tagged proteins were purified with Ni-NTA metal affinity column (Qiagen).
IgG-ELISA
IgG-ELISA was performed according to the method described in Choi et al. (1992) with some modifications. Briefly, antigens were diluted individually in coating buffer (10 mM sodium phosphate, pH 9.3) at a concentration of 5 µg/ml (EXP-1 of 2 µg/ml) and disposed to a 96-well EIA plate (Costar Co., Dover, NH) at 200 µl/well tandemly. The plates were then incubated overnight at 4℃, washed with PBS/0.05% Tween-20 (PBS/Tween) three times, diluted serum (1:100) was added and incubation continued for 2 hrs at 37℃. After washing with the same buffer, 1:1,000 diluted horsedadish peroxidase (HRP)-conjugated goat anti-human IgG antibody (Sigma Chem. Co., St. Louis, MO) was added and the plates were incubated for 2 hrs. After a final washing, 200 µl of substrate solution (1 ml of 1% o-phenylene diamine, 50 µl of 30% H2O2 in 99 ml distilled water) was added. The reaction was stopped by adding 20 µl of 5 N H2SO4 after incubating for 20 min at 37℃. Absorbance was read at 490 nm using an ELISA reader (Dynatech Lab. Inc., Chantilly, VA).
RESULTS
Construction of pQE30 vectors and the expression of His-tagged proteins
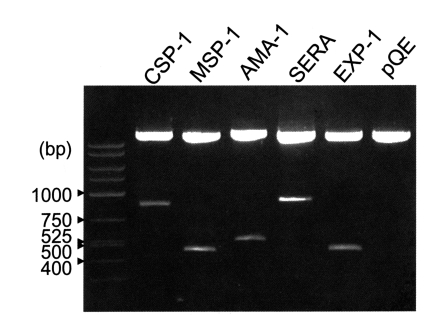
The coding regions for the antigenic domains of CSP-1, MSP-1, AMA-1, SERA, and EXP-1 of P. vivax were amplified by PCR as 774, 456, 506, 867, and 423 bp DNA fragments, respectively. Amplified DNA fragments were cloned into pGEM-T Easy vector and then subcloned into pQE30 vector with the restriction of BamHI and SmaI (Fig. 2).
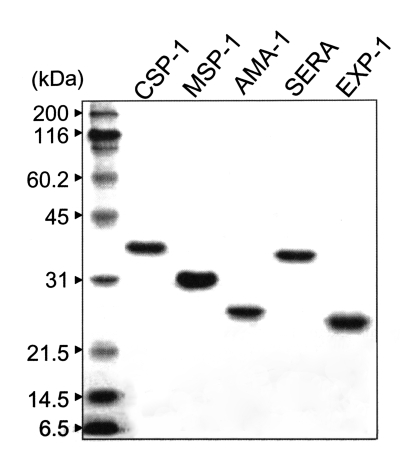
Each protein was expressed as a His-tagged protein in M15 bacteria by IPTG induction with a molecular mass of 36 kDa for CSP-1, 32 kDa for MSP-1, 25 kDa for AMA-1, 35 kDa for SERA, and 24 kDa for EXP-1, respectively, as shown in Fig. 3.
ELISA with patients' sera
To determine the concentration of coating antigens checkerboard ELISA was performed with antigen concentrations of 1, 2, 5, 10, and 20 µg/ml. For all antigens concentrations tested, the 94 negative sera of healthy people reacted at a sufficiently low level, cut-off values were calculated dose-independently. We decided upon cut-off values of 0.20 which represented the mean value + 3 SDs, i.e., 0.08 + 3 × 0.04, for each antigen. Using the 94 positive sera, OD values increased until the concentration of the antigens reached 5 µg/ml, after this value were maintained for CSP-1, MSP-1, AMA-1, and SERA, and at 2 µg/ml for EXP-1.
When applied to patient sera, 122 (80.3%) of 152 vivax malaria cases reacted to at least one antigen, while no reactions were observed for the 128 normal uninfected sera (Table 1). CSP-1 reacted with 60 (39.5%) sera, MSP-1 with 55 (36.2%), AMA-1 with 70 (46.1%), SERA with 53 (34.9%), and EXP-1 with 42 (27.6%).
Screening with the sera of residents in endemic regions
We applied this developed ELISA method to the screening of 3,262 civilian residents in endemic regions near the DMZ. This resulted in 236 positive detections (7.2%) as shown in Table 2. These consisted of 85 positive among 1,202 (7.1%) people in Kanghwa-gun, 27 among 391 (6.9%) in Paju-gun, 19 among 397 (4.8%) in Yonchon-gun of Kyunggi-do, 34 among 558 (6.1%) in Chorwon-gun, and 71 among 714 (10.1%) in Kosong-gun of Kangwon-do.
DISCUSSION
His tagged recombinant proteins coding the antigenic domains of CSP-1, MSP-1, AMA-1, SERA, and EXP-1 of P. vivax were analyzed by ELISA in patients' sera. Each antigen was highly specific and reactive in some sera, but the sensitivity of a single antigen was insufficient due to the complexity of humoral immunity in cases of vivax malaria (Krotoski, 1985). However, when five antigens were combined for detection, we observed that more than 80% of patient sera were found to be positive. Although the sensitivity of the developed needs to be improved, this ELISA containing multiple antigens can be used as an antibody-based detection method.
Although all samples examined were confirmed to be infected with the parasite by blood smear examination, the sera of 30 vivax malaria cases (19.7%) did not react with any antigens of vivax malaria used in this study. These cases were presumed to be of immediate onset, in terms of the disease course, without a prolonged pre-erythrocytic stage, and might have been tested before the production of antibodies to the parasite.
For the screening of residents in endemic regions, sera were collected from people who had not experienced symptoms of vivax malaria infection, during the winter season, to estimate the dormant or prolonged incubation stage of the parasite. A positive rate of 7.2% implies the possibility of symptoms in the population during the following years, moreover, these individuals would serve as human intermediate hosts for the mosquitoes if not treated. The relatively high positive rate in residents of Kosong-gun, Kangwon-do, which are geographically separated by mountains from the former endemic regions in northern Kyunggi-do, suggests expansion of the endemic regions (Lee et al., 2001).
ELISA diagnosis with circumsporozoite and hypnozoite stage antigens will play a critical role in the diagnosis during the prolonged incubation period, especially in cases of vivax malaria. In addition, merozoite antigens in addition to those of the exoerythrocytic stages may provide useful information on the optimal clinical procedure of the diseases. The technique may also be used for epidemiological screening and for safety reasons among blood bank donors. Army soldiers have traditionally served as a large pool of blood donors in Korea, however the overlap of the endemic region and a garrison (Kho et al., 1999) endangers blood supply quality. Repeated mass screening may be helpful in the selection of healthy donors given this situation, we propose a winter screening when the mosquitoes inactive.