AbstractIn the course of Clonorchis sinensis infection, antigens presented to the hosts may be in a close relation to growth of the fluke. The antigenic proteins stimulating IgG antibody production were chronologically identified by immunoblot and localized by immunohistochemical staining. In the early stage of infection until 12 weeks post-infection (PI), antigens were proteins with molecular mass larger than 34 kDa which were derived from the tegument, testes and intrauterine eggs. After 20 weeks PI, antigens recognized were 29, 27 and 26 kDa proteins from the intestine, excretory bladder and reproductive organs. It is suggested that the tegumental proteins are the most potent antigens and the excretory-secretory proteins with middle molecular mass of 26-45 kDa contribute to the high level production of antibodies after 20 weeks of the C. sinensis infection.
INTRODUCTIONAfter infection to the definitive hosts, Clonorchis sinensis finds its way to the niche and grows up to be an adult fluke. Upon an interaction with its host, immune responses are stimulated by antigenic molecules released from C. sinensis. Composition of the antigenic molecules are supposed to be changed from juvenile to adult flukes in accordance to the organogenesis and egg production. As antigen profile changes, the host response or antibody production in this case, will be changed. To develop serodiagnostic antigens for clonorchiasis, it is good to have informations on the potent antigenic molecules and their origins in the fluke in relation to the infection period.
For serodiagnosis of clonorchiasis, the crude preparations of saline extracts and excretory-secretory products of the adult C. sinensis were employed as antigenic materials. The crude antigens showed high sensitivities but did not promise a good specificity. As a way to define antigenic proteins of C. sinensis, the crude worm extracts were fractionated by molecular mass and antigenic proteins were identified to be proteins with low and middle molecular masses from the adult flukes and with high molecular masses from the metacercariae and eggs (Lee et al., 1988). By immunoblotting the crude antigenic preparations with C. sinensis-infected human and animal sera, several proteins were identified to be possible serodiagnostic antigens for human clonorchiasis (Kim, 1994; Hong et al., 1997, 1999). Furthermore, a protein of 7 kDa was proposed as an antigen representing active C. sinensis infection (Kim, 1998). The proteins molecularly cloned from C. sinensis were specific but not sensitive for sera of clonorchiasis patients (Yong et al., 1998: Kang et al., 2001).
In the adult C. sinensis, the antigenic tissues identified by immunostaining using C. sinensis
-infected animal sera, 5 or 10-week infections, are intestinal epithelium, excretory bladder and canals, uterine fluid and eggs, vitelline follicles, testes, and tegument (Chu et al., 1990; Kim et al., 1991).
We are in short for the informations on the immune responses to C. sinensis infections of how the antigenic proteins are presented chronologically, and which tissues as the sources are correspondent to the proteins. This work pursued to collect informations on a chronological profile of antigenic proteins and antigenic localization in tissues of C. sinensis in the course of clonorchiasis.
MATERIALS AND METHODSAdult Clonorchis sinensisMetacercariae of C. sinensis were collected by an artificial digestion from black striped gudgeons, Pungtungia herzi, caught in Gyeongho-gang (river), Sancheong-gun, Gyeongsnagnam-do, and fed orally to a dog. Adult C. sinensis were recovered from liver of the dog at 4-week post-infection (PI).
Rabbit seraThree hundred C. sinensis metacercariae were each fed to the three rabbits. New Zealand White, and the blood was taken from ear veins at 1-6 week intervals over one year. Sera separated from the bloods were kept at -20℃ until used. The number of flukes recovered from the rabbit liver after the last blood collection was 118 in mean vlue.
ImmunoblottingThe adult C. sinensis (0.5 g) were homogenized in 1 ml of phosphate buffered saline (PBS) containing 1% Triton X-100 and lxComplete™ Mini (protease inhibitor cocktail; Boehringer Mannheim, Germany) on ice and kept at 4℃ overnight. The homogenate was spinned at 10,000 g for one hour at 4℃ and the supernatant was saved as a crude antigenic preparation at -20℃. The protein concentration was determined to be 12.7 mg/ml by Lowry method.
The crude antigen was deployed by 13.5% SDS-polyacryamide gel electrophoresis and electrotransferred onto PVDF membrane (Hybond-P, Amersham Life Science, England). The membrane was incubated in rabbit sera at 1:200 dilution and in the secondary antibody, peroxidase-conjugated anti-rabbit IgG antibody (Cappel Co., St. Louis, MO) at 1: 1,000 dilution. Color was developed with a substrate, 4-chloro-1-naphthol (Sigma Immunochemical Co., St. Louis, MO).
Immunohistochemical stainingAdult C. sinensis were recovered from the liver of a dog 13 weeks after the initial metacercarial infection and fixed in 10% neutral formalin. The fixed flukes were processed for the paraffin block and ribbons according to the conventional method. The ribbons were stained with the Zymed LABSA™ system according to the manufacturer's instruction (Zymed Lab. Inc., South San Francisco, CA). After rinsing with PBS, the ribbons were incubated for 30 min each in rabbit sera diluted at 1:100-1:500 and in a biotinylated second antibody at 1:2,000 dilution, and in streptavidin-peroxidase (HRP) conjugate solution for 20 minutes. The ribbon was visualized in a substrate, 3-amino-9-ethylcarbazol, and counterstained with hematoxylin.
RESULTSProfile of antigenic proteins in immunoblotsIn the course of experimental clonorchiasis in rabbits, antigenic proteins reacting to IgG antibodies produced were as followings (Fig. 1). At 3-week PI, proteins with higher molecular mass than 42 kDa began to be recognized by antibodies, of which stronger antigens were 50, 69 and 96 kDa proteins. At 4-week PI, proteins of 40, 39, and 38 kDa produced strong signals. At 8 week PI, antibodies were reactive to proteins of 36, 34, 30, and 10 kDa. A protein of 29 kDa appeared as the antigen at 12-week PI. From 20 week PI, 29, 27 and 26 kDa proteins were added as distinct antigens to the antigenic protein pool, and 16 and 19 kDa proteins to the pool as less distinctive ones. Of them, proteins with molecular masses between 26 and 45 kDa became increasingly more potent antigens and continued to remain as major antigens for over one year.
Localization of antigenic proteins in tissuesIn the immunohistochemical staining of C. sinensis, those stained were tegument, subtegument, interstitial tissue of testes, and uterine fluid and eggs as early as 3-week PI (Fig. 3). Of the distal stretch of genital ducts in front of the ventral sucker, the female genital duct, the metraterm, was stained, but the male genital duct was not (data not shown). At 4-week PI, the intestinal epithelium and the wall of excretory bladder were stained lightly (Fig. 4), while the tegument was stained moderately. At 8-week PI, the ovary and wall of seminal receptacle, inner surface of the ventral sucker were stained lightly. The staining of subtegument, intestinal epithelium and excretory bladder were enhanced to a moderate degree. From 12-week PI, the interstitial tissue of vitelline follicles and the mesenchymal tissue of body were stained lightly (Table 1).
In the infection period of 20- to 40-week PI, the Intestinal epithelium, excretory bladder, testes and seminal receptacle retained moderate stainings (Fig. 5). The uterine fluid produced moderate staining. Accordingly the egg shell surface was coated with the moderate staining color. Embryos in the intra-uterine eggs were stained moderately from 32-week PI (Fig. 6). The tegument showed the strongest staining of the C. sinensis tissues between 32 and 40 weeks PI (Fig. 7), and decreased thereafter. Stainability of ovary was not consistent after 32-week PI (Table 1).
In stage of the Infection after 48-week PI, intensity of staining in the tegument, ovary, seminal receptacle and intra-uterine eggs was diminished by one grade. Tegument and secretory-excretory organs were strongly localized with antigens presented to the host in the course of C. sinensis-infection.
DISCUSSIONOf the antigenic proteins, the higher mass antigens were identified specific to the metacercariae and eggs of C. sinensis (Lee et al., 1988). Since the egg was not produced in the juvenile flukes, the antigenic proteins reacting to antibodies in sera of the earlier infection, distinguished by higher molecular masses, seems to be derived from the tegument. Antibodies reactive to the tegument became stronger as the fluke grew up and displayed the strongest signal in the later stage of the infection in this study. This suggested that the shedded tegumental material was increased in proportion to an increase of body surface of the metacercaria to mature fluke of C. sinensis (Kim, 1995) and provided antigenic molecules (Chu et al., 1990). Of the characterized molecules from C. sinensis, phosphoglycerate kinase and 28 kDa glutathione S-transferase were also localized in the tegument (Hong et al., 2000; Kang et al., 2001).
The epithelial cells of the intestine and excretory bladder have been recorded as the solid and strong antigenic tissues in C. sinensis (Chu et al., 1990; Kim et al., 1991). As an exporter and regulatory barrier, the epithelial cells may hold or contain relatively a large amount of the antigenic molecules, which accordingly present as highly antigenic. The antigenic proteins showing serodiagnostic usefulness were identified in the excretory-secretory products prepared with culture media of adult C. sinensis (Sun and Gibson, 1969). The potent antigenic proteins have middle and low molecular masses ranging 7-43 kDa and react to IgG antibodies (Kim, 1994, 1998; Hong et al., 1999). Among them, the 7 kDa protein was proposed as a possible antigen for serodiagnosis of active clonorchiasis (Kim, 1998). It is informative that the antigenic proteins with middle-ranging molecular masses in the excretory-secretory products supported the high titers of IgG antibodies in middle and later stage of C. sinensis-infections
.
The genital organs such as testes, ovary, vitelline follicles, and uterine fluid and eggs have been identified using immunological staining methods to contain potent antigenic proteins (Chu et al., 1990; Kim et al., 1991). In the final hosts, the reproductive organs of C. sinensis make appearance around 6 days PI (Kim, 1995) and the intra-uterine eggs are produced at 15 days PI (Lee et al., 1973). IgG antibodies reacting to genital organs were produced and enhanced with a latency of 1-4 weeks from the antibody produced toward the tegumental tissues. This latency period of antibody production is in accord with time lag between the organogenesis and egg production of C. sinensis and the metacercarial infection. In antigen preparations with adult C. sinensis, it was technically difficult to separate the secretions of genital organs from the excreted-secreted materials, and reported thereby as ingredients. Those proteins proposed as potential serologic antigens are, as are in this work, of proteins ranging middle to lower molecular masses (Lee et al., 1988; Kim, 1994). An antigenic protein, with molecular mass of 38 kDa and specific to C. sinensis-infected sera, was localized in vitelline follicles (Yong et al., 1998; Yang et al., 2000). The genital organs of C. sinensis are here recognized as the third portion of antigenic tissues and secreted a part of antigenic proteins with middle and lower molecular masses. It is probable that the genital secretions increase an antibody production in the middle period of C. sinensis-infections.
The eggs of tissue-invading flukes secret strong antigenic proteins which recruit inflammatory cells and form granulomas (Kang et al., 1991; William et al., 2001). The C. sinensis egg has been identified to contain antigenic proteins ranging low to high molecular masses (Lee et al., 1988; Kim et al., 1991; Hong et al., 2000; Kang et al., 2001). Therefore, it is suggested that the egg proteins deserve further analysis toward development of serodiagnostic antigens.
REFERENCES1. Chu BD, Rim HJ, Kim SJ. A study on the body fluid antigen of Clonorchis sinensis using immunogold labeling method. Korean J Parasitol 1990;28:11-23.
2. Hong ST, Kho WG, Lee M, Lee JS, Lee SH. Immunoblot patterns of clonorchiasis. Korean J Parasitol 1997;35:87-93. PMID: 9241982.
3. Hong ST, Lee M, Sung NJ, Cho SR, Chai JY, Lee SH. Usefulness of IgG4 subclass antibodies for diagnosis of human clonorchiasis. Korean J Parasitol 1999;37:243-248. PMID: 10634040.
4. Hong SJ, Seong KY, Sohn WM, Song KY. Molecular cloning and immunological characterization of phosphoglycerate kinase from Clonorchis sinensis. Mol Biochem Parasitol 2000;108:207-216. PMID: 10838223.
5. Kang SY, Ann IY, Park CY, et al. Clonorchis sinensis: Molecular cloning and characterization of 28-kDa glutathione S-transferase. Exp Parasitol 2001;97:186-195. PMID: 11384162.
6. Kang SY, Kong Y, Cho SY. Component proteins in crude extract of adult Paragonimus westermani purified by immunoaffiniiy chromatography using monoclonal antibodies. Korean J Parasitol 1991;29:363-369.
7. Kim J. Image analytical observation on the growth and development of Clonorchis sinensis in rats. Korean J Parasitol 1995;33:281-288. PMID: 8591005.
8. Kim J, Chai JY, Kho WG, Cho KH, Lee SH. Immunohistochemical study on the antigenicity of each organ structure of Clonorchis sinensis. Korean J Parasitol 1991;29:21-29.
9. Kim SI. Immunoreaction between excretory-secretory antigens and specific antibodies of Clonorchis sinensis before and after praziquantel treatment in experimentally infected rabbits. Korean J Parasitol 1994;32:35-42. PMID: 8167106.
10. Kim SI. A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol 1998;36:37-45. PMID: 9529862.
11. Lee OR, Chung PR, Nam HS. Studies on the immunodiagnosis of rabbit clonorchiasis 2. Immunoaffinity purification of whole worm antigen and characterization of egg, metacercaria and adult antigns of Clonorchis sinensis. Korean J Parasitol 1988;26:73-86.
12. Lee WK, Lee KM, Lee OR, Choi WY. Host-parasite relationship in trematodes. I. Susceptibility and development of Clonorchis sinensis in rabbits. Korean J Parasitol 1973;11:76-82.
13. Sun T, Gibson JB. Antigens of Clonorchis sinensis in experimental and human infections. Am J Trop Med Hyg 1969;18:241-252. PMID: 4975976.
14. William S, Sabra A, Ramzy F, et al. Stability and reproductive fitness of Schistosoma mansoni isolates with decreased sensitivity to praziquantel. Int J Parasitol 2001;31:1093-1100. PMID: 11429173.
15. Yang HJ, Park SJ, Im KI, Yong TS. Identification of a Clonorchis sinensis gene encoding a vitellaria antigenic protein containing repetitive sequences. Mol Biochem Parasitol 2000;111:213-216. PMID: 11087931.
16. Yong TS, Yang HJ, Park SJ, Kim YK, Lee DH, Lee SM. Immunodiagnosis of clonorchiasis using a recombinant antigen. Korean J Parasitol 1998;36:183-190. PMID: 9755589.
Fig. 1An immunoblot of adult Clonorchis sinensis crude antigen against a rabbit serum. The sera were collected from the rabbit over one year after the metacercarial infection. 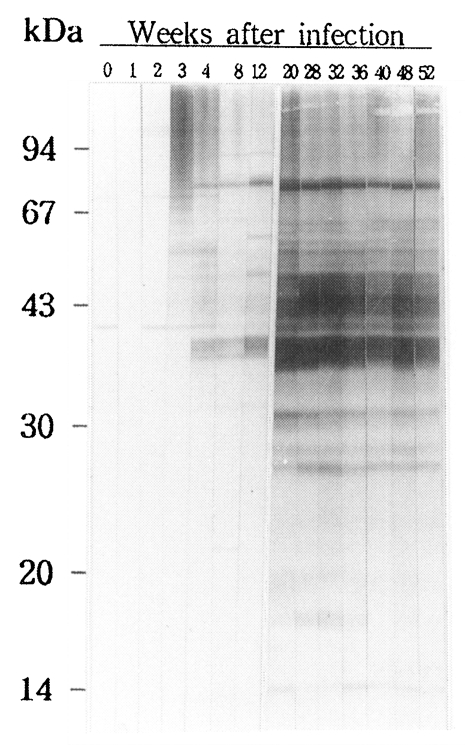
Figs. 2-7Immunohistochemical staining of adult Clonorchis sinensis with the rabbit sera of C. sinensis-infection. Fig. 2. Control stained with a pre-infection serum. In, intestine; Tg, tegument; Ts, testes. Fig. 3. A serum of 3-week post-infection (PI) produced light red color in tegument and in uterus (Ut) and eggs. Fig. 4. The intestinal epithelium and excretory bladder (EB) were stained lightly and the tegument was moderately by a serum of 4-week PI. Fig. 5. Stained with a serum of 28-week PI. Note the stains in the vitelline follicles (VF) and wall of seminal receptacle (SR). The mesenchymal tissue was recognized to be a light staining. Fig. 6. The epithelium of miracidium in intra-uterine egg was stained moderate by a serum of 28-week PI. Bar is 50 µm. Fig. 7. A serum of 32-week PI produced a strong stain in the tegument and moderate ones in testes and intestinal epithelium. The mesenchymal tissue was stained by a moderate degree. Bars are 100 µm. 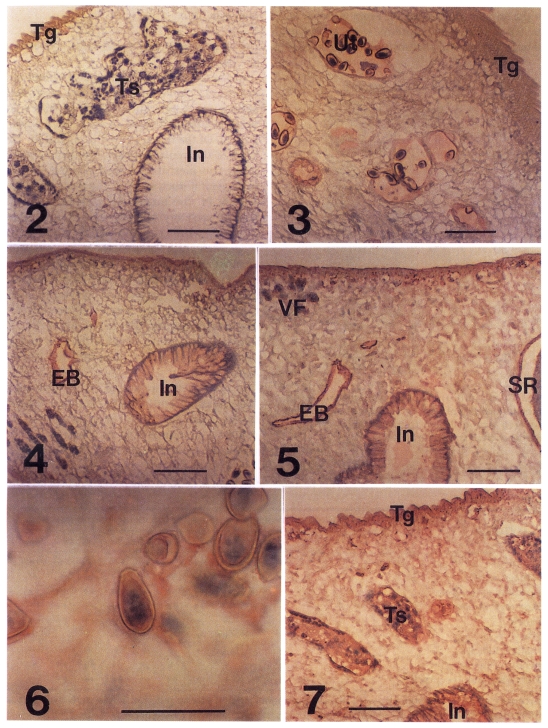
Table 1.Immunohistochemical localization of antigens in tissues of adult Clonorchis sinensis according to infection period
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||