INTRODUCTION
Human sparganosis, a helminthic disease caused by tissue invading Spirometra mansoni plerocercoid, is characterized by a granulomatous lesion. Although the infection occurs worldwide, it is more frequently found in East and Southeast Asia. The larva lodges usually in the subcutaneous tissues and sometimes invades the central nervous system causing neurologic diseases (Chang et al., 1987, 1992). Clinically, one or more lesions are found in different parts of the body. Therefore, preoperative presumption of a local lesion as due to sparganosis is not always feasible unless presenting with typical symptoms such as a migratory nodule. Although clinical need for antibody tests is not high, the antibody test is a useful tool for epidemiological survey in susceptible population (Kong et al., 1994a) and for routine screening of neurological patients living in endemic areas (Chang et al., 1987, 1992).
Antibody tests for sparganosis appear to be sensitive and specific in surgical cases when examined by enzyme-linked immunosorbent assay (ELISA) (Kim et al., 1984) or by chemoluminescent-ELISA (Nishiyama et al., 1994). The IgG-binding antigenic proteins have been purified as trypsin-like and chymotrypsin-like proteases in the crude extracts of the plerocercoid (Choi et al., 1988; Cho et al., 1990; Morakote and Kong, 1992; Kong et al., 1994b). On the contrary, cysteine proteases at 27 and 53 kDa in the extracts were found to be IgE binding antigens in human sparganosis (Kong et al., 1994b, 1997). Despite these progress on the antigen characterization and serodiagnostic techniques, very few studies have been undertaken in sparganosis especially on the antibody responses in some aspects. For example, antibody responses in early infection stages and subsequent isotype changes are awaiting to be elucidated. The present study observed IgG antibody responses in early experimental murine sparganosis and IgG subclass responses in human sparganosis.
MATERIALS AND METHODS
Parasite, antigen and serum samples used
The spargana were collected from naturally infected terrestrial snakes, Elaphe rufodorsata. After washing with physiological saline, 5 g of spargana in 20 ml Tris buffered saline (50 mM, pH 7.5) containing 0.1 mM EDTA, was ground twice with teflon-pestle tissue homogenizer. The homogenate was centrifuged twice at 700 g for 5 minutes. Supernatants were obtained by centrifugation again at 20,000 g for 1 hr. All procedures were done at 4℃.
Outbred ICR mice were fed five scolices of spargana. After the infection, 3 mice were killed by heart puncture to collect serum samples on 2, 4, 7, 14, 21, 28, 35, 42, 56 and 67 days, respectively. By autopsy, the mice were confirmed to be infected with 2-5 spargana of different length.
A total of 69 sera from patients with sparganosis were selected from our sera bank. The patients were diagnosed either by surgical removal of the worm or by antibody positive reactions by ELISA (Kim et al., 1984). Twenty sera from healthy people who denied possible exposure to helminthic infection sources were used as control.
IgG immunoblot for sera of experimental mice
IgG immunoblots for sera of experimental mice was done by the method described by Yang et al. (1998). Proteins in the crude extracts were resolved by SDS-PAGE, and transferred to polyvinylidene difluoride (PVDF) membrane. Blots were incubated overnight with control and infected mice sera, diluted at 1:100. Peroxidase conjugated anti-mouse IgG (1:1000 dilution, heavy- and light-chain specific, Cappel) was reacted for two hr. The blots were developed using 4-chloro-l-naphthol chromogen.
Generation of monospecific antibody against 36/31 kDa proteins
The monospecific antibody against the 36 kDa chymase of the sparganum was raised in a rabbit which was immunized with 10 µg of the 36 kDa proteins mixed with Freund's adjuvant (Sigma, St. Louis, MO, USA) 3 times at 2 weeks interval. The proteins were purified by the methods described previously using gelatin affinity chromatography (Kong et al., 1992). The antibody was stored at -70℃ until
use.
Differential immunoblot
Differential immunoblot was undertaken to confirm character of the main antigenic proteins at 36-26 kDa as chymase of the sparganum and its degradation products. The blocking antibody used in the differential immunoblot was the rabbit monospecific antibody raised as above. The proteins of sparganum resolved by SDS-PAGE were transfer-blotted to PVDF microporous membranes (Millipore, Bedford, MA, USA). The strips were reacted with anti-36 kDa rabbit sera and unlabelled anti-rabbit IgG (Cappel, Cochraville, PA, USA). Subsequently, the strips were reacted with the experimental mice sera and peroxidase conjugated anti-mouse IgG (Cappel). The immunoreaction was visualized by 4-chloro-l-naphthol chromogen (Sigma).
Subclass ELISA and immunoblot
ELISA for specific IgG subclass was done as Yang et al. (1998) described. In brief, microtiter plates (Costar, Cambridge, MA, USA) were coated overnight with the crude extracts (protein concentration 2.5 µg/ml in carbonate buffer, pH 9.6) and reacted with patient sera in dilution of 1:100 in phosphate buffered saline for 2 hr. Mouse anti-human IgG subclasses were reacted for 2 hr in the following dilutions; 1:500 for anti-human IgG1 (JL512) and 1:1000 for anti-human IgG2 (GoM1), IgG3 (ZG4), and IgG4 (RJ4) (Immunotech, Marseille, France). Peroxidase conjugated goat anti-mouse IgG (Fc specific) was diluted at 1:500 and further reacted for 2 hr. Color reaction was developed with o-phenylene diamine (Sigma) and absorbance was read at 490 nm.
For IgG subclass immunoblot, the method described by Yang et al. (1998) was followed. The proteins were resolved by SDS-PAGE, and transferred to PVDF membrane. Blots were incubated overnight with patient sera, diluted at 1:100. Peroxidase conjugated anti-human IgG (1:1000 dilution, heavy- and light-chain specific, Cappel), and monoclonal antibodies against human IgG subclasses (G1, G2, G3 and G4, respectively, Zymed, San Francisco, CA, USA) were diluted at 1:750, 1:1000, 1:1000 and 1:1000, respectively, and incubated for 2 hr. The blots were developed as described above using 4-chloro-l-naphthol chromogen.
RESULTS
Early IgG antibody responses in experimental mice
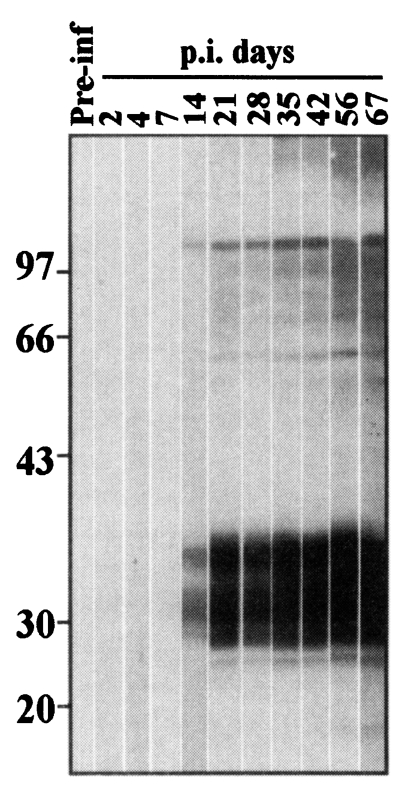
As shown by immunoblot (Fig. 1), experimental mice began to react with the crude extracts from 14 days after infection. Relatively weak reactions were observed against two components in the crude extracts of sparganum such as 103 kDa and 36-26 kDa which reacted stronger thereafter. Since 21 days after infection, antigenic proteins at 100-70 kDa and at approximately 60 kDa also reacted with the infection sera. On 35 days and thereafter, antigens around 200 kDa reacted with the infection sera.
To confirm character of the main antigenic proteins at 36-26 kDa as chymase of sparganum at 36 kDa and its degradation products, differential immunoblots were undertaken. The multiple reacting bands in 36-26 kDa of the crude extracts (panel A, Fig. 2) disappeared when rabbit anti-chymase monospecific serum was treated before the reaction with sera of infected mice (Fig. 2 panel B). Antigenic components larger than 60 kDa continued to react with the sera of infected mice.
IgG subclass responses in patients sera
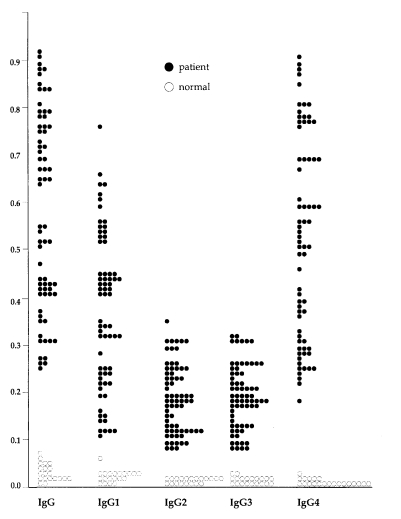
In 69 sera from patients with sparganosis, mean and standard deviation of absorbance of the total IgG and IgG subclass were: 0.58 ± 0.21 for total IgG, 0.37 ± 0.17 for IgG1, 0.19 ± 0.07 for IgG2, 0.19 ± 0.07 for IgG3 and 0.52 ± 0.21 for IgG4, respectively. In 20 sera of normal control, they were: 0.02 ± 0.01 for total IgG, 0.03 ± 0.01 for IgG1, 0.02 ± 0.01 for IgG2, 0.02 ± 0.01 for IgG3 and 0.01 ± 0.01 for IgG4. Difference of the absorbance between sparganosis and control groups were statistically significant (p < 0.005) by Student t-test. Distribution of the absorbance in individual cases were shown in Fig. 3.
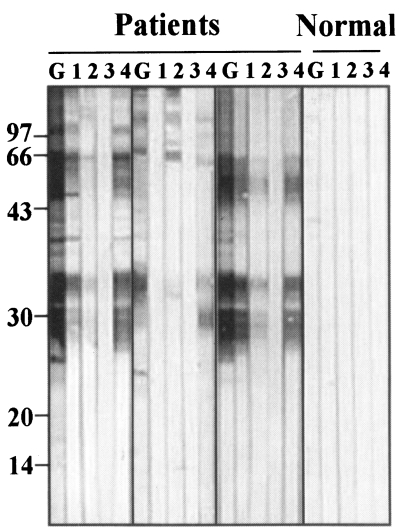
Representative immunoblots findings of antigenic proteins, reacted with IgG subclass antibodies, were shown in Fig. 4. Antigenic bands reacting with total IgG and IgG4 were almost identical. Main reactions were shown at 36-26 kDa and 100-70 kDa bands. Reactions against IgG1 were weaker than those against total IgG and IgG4 although they were observed at identical bands. IgG2 and IgG3 antibodies showed weak or no reactions with the crude extracts of sparganum.
DISCUSSION
In the experimental mice sparganosis, antigenic bands at 36-26 kDa and at 103 kDa were eliciting IgG antibody reactions as early as two weeks. Another antigenic band with high molecular mass at approximately 200 kDa also elicited the antibody reactions after five weeks of infection. As confirmed in the differential immunoblot, the 36-26 kDa antigens are chymase and its degradation products of sparganum. Sparganum chymase has been known to show self-digestion activities (Kong et al., 1994b). Smudging reactions at 36-26 kDa in the immunoblot make it unclear whether 28 kDa cysteine protease of sparganum (Song et al., 1993; Kong et al., 1994c) is antigenic in the early sparganosis. The cysteine protease, however, was confirmed not to elicit IgG antibody reaction because the differential immunoblot did not reveal any positive reaction around 28 kDa in this study.
Antigenic bands at 103 kDa and at approximately 200 kDa are probably trypticases of sparganum. Kong et al. (1994b) purified the two proteolytic enzymes by chromatographic steps and found that they are highly antigenic in human sparganosis. The two trypticases as well as chymase were excreting outside the sparganum and are considered to facilitate migration of the larva by degrading host extracellular matrices such as collagen, fibronectin and myelin basic protein (Kong et al., 1994b).
In the present study of experimental sparganosis, the proteolytic enzymes at 36 kDa/103 kDa and at approximately 200 kDa showed about 3-week interval in eliciting antibody reactions. The interval between them can not be properly explainable. A presumption is that the antigenic trypticase at approximately 200 kDa are produced mainly at the tail of sparganum which is cut off during oral infection and sprouting again from the invading scolex. However, this presumption should be confirmed by differential examination of the enzyme activities in scolex and tail of the plerocercoid.
Human sparganosis is a chronic infection of long duration (Cho et al., 1975). In fact, however, the exact period of infection can hardly be assessed in individual cases because some modes of daily life such as drinking untreated water can give rise to the infection. Many of raw meat eaters have histories of eating different animal meats, and it is frequently difficult to determine which of the raw meat has been the source of infection. Therefore, the time when actual infection occurred can hardly be determined in most human sparganosis. In addition, most cases manifest no symptoms especially in the early stages. In this respect, Weinstein et al. (1954) recorded a case manifesting a migrating subcutaneous mass developed within two months after eating raw snakes. This exceptional case may be one of a few cases manifesting early human sparganosis. Even in these early cases, serological diagnosis of the infection seems possible because the antigenic epitopes at 36/31 kDa recognized the antibodies produced as early as two weeks post-infection as seen in this study.
In human infection sera, IgG4 subclass was found to be the main antibody reacting with the sparganum antigens. IgG1 antibody showed intermediate reactivity. Reactivities of IgG2 and IgG3 antibody were weak. IgG4 reaction seems to be predominant because most human sparganosis are chronic (Yang et al., 1998). However, it should be noted that specific IgG reaction was not directed to cysteine protease of sparganum which are eliciting IgE reaction (Kong et al., 1994c). Because total IgG and IgG4 subclass reacted with the same antigenic epitopes, we can surmise that IgG4 did not react with IgE eliciting cysteine protease of sparganum either. If it is true, this means that the IgG4 subclass is not playing a role of blocking antibody in the case of sparganosis. This presumption deserves further studies.
Together with the findings of Morakote and Kong (1993), which described specificity of the enzyme against tissue nematode infections, this study confirms that 36 kDa chymase of S. mansoni plerocercoid and its degradation products are also important antigen for IgG antibody responses in early stage of experimental sparganosis and for IgG subclass reactions in human sparganosis.