Giardia lamblia is a worldwide causative agent of diarrheal disease (Adams, 1991). Infection occurs with ingestion of cysts and is followed by excystation to trophozoites in the small intestine of a host (Craun, 1990).
Since G. lamblia is believed to have a broad host range including humans and animals (Kulda and Nohynkova, 1995), inter-species infections may be possible. The traditional grouping of Giardia, based only on the morphology, has hampered elucidation of significant genetic differences as well as the transmission modes between diverse Giardia strains. The availability of axenic Giardia strains from diverse sources may be an essential and fundamental step in these studies. Careful comparisons between diverse Giardia strains should shed light on its zoonotic potential as well as the mechanism of virulence.
In this report, we established two G. lamblia isolates, K1 and K2, as axenic cultures for the first time in Korea and these two isolates were grouped by two different genetic analyses.
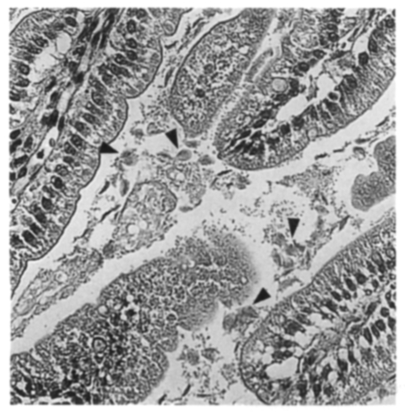
In 1998, fecal samples of outpatients who visited Severance Hospital, Yonsei University College of Medicine, were examined under a light microscope for the presence of Giardia cysts. Cysts were repeatedly washed, purified on sucrose density gradient centrifugation (Bingham and Meyer, 1979), and then stored at 4℃ for a week. About 10,000 cysts from two cyst-passers were orally administered to three suckling Mongolian gerbils (Meriones unguiculatus). At day six post-inoculation, the small intestines of the gerbils were examined for Giardia trophozoites. Motile trophozoites were found in all of the gerbils, especially in the proximal part of the small intestine. Haematoxylin-eosin staining of the infected tissue revealed numerous Giardia trophozoites as free or attached forms to the microvilli of the intestine (Fig. 1).
In vitro cultures were initiated by inoculating the trophozoites in modified TYI-S-33 medium (Keister, 1983). To inhibit the growth of bacteria, streptomycin and penicillin G (Sigma, St. Louis, USA) were added at the onset of the primary culture at the concentrations of 400 IU/ml and 200 µg/ml, respectively. Then, the addition of antibiotics was omitted after 1 month of cultivation. Two isolates originated from two cyst-passers were axenically maintained for more than 10 months and named K1 and K2.
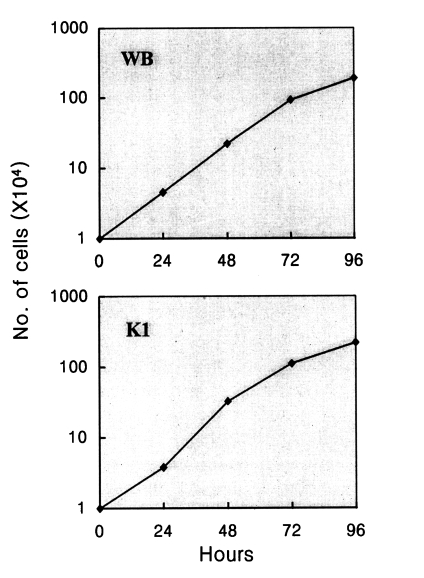
To characterize the growth pattern of the K1 isolate, tubes containing 10 ml of medium were inoculated with 104 trophozoites from logarithmic phase cultures. Every 24 hr, the tubes were chilled on ice for 10 min to detach adherent trophozoites. The number of cells was determined by counting on a haemocytometer. Fig. 2 represents the growth curves for WB and K1 isolates. According to the formula described by Boreham et al. (1984), the generation times of K1 and WB were calculated as 14.2 and 14.3 hr, respectively. These generation times are typical for "medium-rate grower" according to Karanis and Ey (1998). Another Korean isolate, K2, was found to grow at a similar rate with K1 (data not shown).
Genetic characterization of K1 and K2 was performed using PCR to amplify a segment of Giardia SSU-rDNA (for small subunit ribosomal DNA) and two parts of tim gene (for triose phosphate isomerase). Genomic DNA of K1 and K2 trophozoites were purified by SDS lysis method (Hopkins et al., 1997). Samples were lyzed by five cycles of freeze-thawing, and resuspended in 600 µl of lysis buffer (pH 7.5) containing 1% SDS, 20 mM Tris-HCl, 20 mM EDTA, and 50 mM NaCl. After incubation at 56℃ for 3 hr, DNA was subjected to phenol/chloroform/isoamylalcohol and chloroform extractions, followed by RNase treatment. Samples were then treated with chloroform and precipitated with ethanol.
Five to fifty ng of these genomic DNA were used as a template to amplify a 292 bp region of the 5' end of the SSU-rDNA using following primers: sense primer, RH11 (nt. 1-18) 5'-CATCCGGTCGATCCTGCC-3'; antisense primer RH4 (nt. 268-292) 5'-AGTCGAACCCTGATTCTCCGCCAGG-3' (Hopkins et al., 1997). The PCR reaction was performed in a Perkin-Elmer thermocycler (Geneamp PCR system 9600). The reactions for amplification of genomic DNAs were performed as follows: denaturation at 96℃ for 20 sec, hybridization at 59℃ for 20 sec, and chain polymerization at 72℃ for 30 sec. The reactions were allowed to proceed for 35 cycles, and the reaction was linked to a final polymerization step of 7 min at 72℃. Upon agarose gel electrophoresis, the 292 bp PCR fragments were extracted by Qiaex II kit (Qiagen, Madison, USA) and cloned to pGEM easy vector (Promega, Hilden, Germany). Sequencing reactions were performed using Taqtrack system (Promega) with either T7 or SP6 primers. Fig. 3 shows the nucleotide sequences of the 292 bp segment of SSU-rDNA in K1, and K2 along with Group 1 to 4 described by Hopkins et al. (1997). While K1 showed 5 bases difference from Group 1 Giardia, K2 exhibited exactly the same sequence as Group 1 Giardia. Despite the difference in nucleotide sequence between K1 and Group 1 Giardia, phylogenetic analysis using PAUP 3.1.1 indicated that such difference was not significant (data not shown). Therefore, this results suggest that both K1 and K2 isolates belong to Group 1 of Hopkins et al. (1997).
Due to the indiscrepancy of genetic grouping of Giardia among research groups around the world, we performed a different genetic analysis of K1 and K2. Using an alignment of the tim genes, three genotypes defined initially by Nash (1992) had been re-examined by Baruch et al. (1996). The nucleotide sequences of tim gene of three Nash's groups demonstrated similarities between group 1 and 2 (1% nucleotide divergence) and an 18% divergence between group 1 and 3 (Lu et al., 1998). We also analyzed the tim gene of K1 and K2 isolates through PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism). Two regions of the tim gene were amplified from K1 and K2 genomic DNAs. Primers 7493 (5'-GCAGAATGTGTACCTAGAG GGG-3': nt. 192-213) and 5945 (5'-TAGTCTCC GAGCTCCTTCTGG-3': nt. 1004-984) were used to amplify a 812 bp DNA fragment of the 3' end of tim, whereas primers 4131 (5'-ATGCCTGCTCGTCGCCCCTTC-3': nt. 1-12) and 4130 (5'-CACTGGCCAAGCTTCTCGCAG-3': nt. 683-663) were employed to produce a 683 bp DNA fragment corre-sponding to the 5' end of the tim gene. Samples were initially denatured at 95℃ for 5 min and followed by 35 cycles of amplification (1 min denaturation at 95℃, 30 sec annealing, and 1 min extension at 72℃) and a final 5 min extension at 72℃. Annealing temperatures were between 45℃ and 55℃.
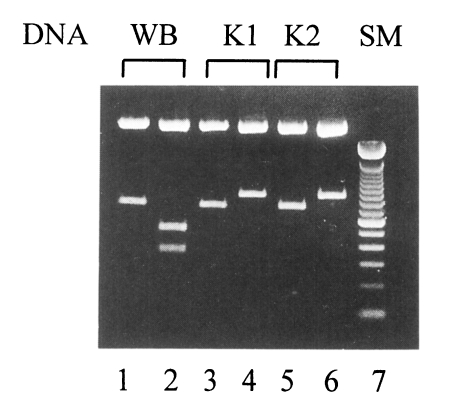
Depending on the presence of the restriction sites on these two tim fragments, Giardia can be easily categorized into one of three Nash's groups (Nash, 1992). Group 1 Giardia has no XhoI site on its 683 bp tim fragment whereas there is a HindIII site on its 812 bp tim fragment. In case of group 2 Giardia, there is neither HindIII nor XhoI site on these fragments. On the contrary, group 3 Giardia has a HindIII site on its 812 bp DNA while a XhoI site is present on its 683 bp DNA. As shown in Fig. 4, WB isolated from an Afganistan human (Nash et al., 1985) and our two isolates were characterized on the basis of this analysis. As a representative of group 1, WB strain (ATCC 30957) was expected to have a HindIII site on its 812 bp tim fragment. For WB, being digested with HindIII, two fragments of 478 and 334 bp were visualized on a 1.5% agarose gel. On the other hand, there was no XhoI site observed in 683 bp tim DNA as expected. The 683 bp tim DNA fragments of both K1 and K2 were intact with XhoI digestion as same as WB. Unlike WB, there was no HindIII site in the 812 bp DNA fragments of K1 and K2. This result implied that two Korean isolates, K1 and K2, belong to Nash's group 2 defined by Baruch et al. (1996).
In this study, two axenic Giardia strains were established in Korea by infecting suckling Mongolian gerbils with the human cysts. Using the in vitro culture, K1 was characterized as a "medium-rate grower" due to its growth pattern. Despite some differences in genetic analysis with SSU-rDNA sequence, both K1 and K2 were found as members of Hopkins' group 1. However, via different genetic analysis using PCR-RFLP of the tim gene, K1 and K2 were found to belong to Nash's group 2. Therefore, Nash's group 2 can not be a separate group from Hopkins' group 1. Since only 1% divergence was observed in the tim coding sequence between Nash's group 1 and 2 (Lu et al., 1998), it is likely that Nash's group 1 and 2 comprise the Hopkins' group 1. However, more studies are needed in the future to find out how these groupings can be integrated to give a general picture about the classification of diverse Giardia strains.