Molecular Variation and Distribution of Anopheles fluviatilis (Diptera: Culicidae) Complex in Iran
Article information
Abstract
Anopheles fluviatilis James (Diptera: Culicidae) is one of the known malaria vectors in south and southeastern Iran. Earlier ITS2 sequences analysis of specimens from Iran demonstrated only a single genotype that was identical to species Y in India, which is also the same as species T. We identified 2 haplotypes in the An. fluviatilis populations of Iran based on differences in nucleotide sequences of D3 domain of the 28S locus of ribosomal DNA (rDNA). Comparison of sequence data from 44 Iranian specimens with those publicly available in the Genbank database showed that all of the 28S-D3 sequences from Kazeroun and Khesht regions in Fars Province were identical to the database entry representing species U in India. In other regions, all the individuals showed heterozygosity at the single nucleotide position, which identifies species U and T. It is argued that the 2 species may co-occur in some regions and hybridize; however, the heterozygosity in the 28S-D3 locus was not reflected in ITS2 sequences and this locus for all individuals was identical to species T. This study shows that in a newly diverged species, like members of An. fluviatilis complex, a single molecular marker may not be sufficiently discriminatory to identify all the taxa over a vast geographical area. In addition, other molecular markers may provide more reliable information for species discrimination.
INTRODUCTION
Anopheles fluviatilis James is responsible for transmission of malaria along the foothills of the Zagros Mountains that stretches from south to southeast in Iran [1-3]. Various biological studies in Iran demonstrated differences among populations of this species in feeding preference, resting behaviors, and infection rates, which suggested the existence of different biological forms [4,5]. Cytotaxonomic studies of fixed inversions in polytene chromosomes arm 2 of Indian specimens identified 3 reproductively isolated cryptic species in the An. fluviatilis complex that were designated as S, T, and U [6]. Later, 2 putative species of X and Y were identified in Orissa State, east-central India, based on differences in the second internal transcribed spacer (ITS2) of ribosomal DNA (rDNA) [7]. Comparison of the ITS2 sequence in Iranian specimens from south and southeastern Iran, including Fars, Sistan va Baluchestan, Kerman, Bushehr, and Hormozgan, showed that they were all homologous to the published ITS2 sequences of species Y (presumably species T) from India [5,8]. Cytotaxonomic studies on specimens from Iranshahr, Sistan va Baluchestan Province, showed that they were synonymous with species T [5]. Another ITS2 sequence from Minab, Hormozgan (N. D. Djadid, Unpublished data) demonstrated 1.87% divergence from other Iranian sequences, which was designated as form V by Chen et al. [9] and later as species V by Azari-Hamidian [10]. Recently, 3 members of the An. fluviatilis complex; S, T, and U were identified based on differences in nucleotide sequences within the D3 domain of the 28S (28S-D3) rDNA. Sequencing data for 375 base pair products showed that species S differed from species T and U by 3 nucleotide bases and species T and U differed from each other by only 1 nucleotide base [11]. Limited 28S-D3 sequences from Hormozgan and Sistan va Baluchestan showed that while they shared the closest relationship with species T and U, they differed only at a single base position that characterizes the 2 species in India [9].
Based on variations in the D3 domain of the 28S region, we report the presence of 2 haplotypes and their approximate distribution among An. fluviatilis populations in Iran. We also discuss the possibility of the occurrence of An. fluviatilis form V in Iran.
MATERIALS AND METHODS
Mosquitoes
Adult and larval mosquitoes were collected from south and southeastern Iran, including Fars, Hormozgan, Kerman, and Sistan va Baluchestan Provinces (Table 1), during March to November 2002 and January to April 2003. The Latitude and longitude coordinates of collection sites were obtained from the website http://itouchmap.com/latlong.html. Collection of adult mosquitoes were conducted using mouth aspirators during the day from indoors, including human dwellings, cattle sheds, and shelter pits, and during the night by animal bait and human volunteers. The larvae were collected using a dipper, along the margin of clear running streams. The larvae were reared to adults in an insectarium at 30 ± 2℃, 75 ± 10% humidity, and 12 hr light-dark cycle. Emerged adults were mounted on pins and identified using a morphological key [12].
Sequencing of DNA
Genomic DNA was extracted from whole bodies of individual mosquitoes using the phenol-chloroform protocol of Ballinger-Crabtree et al. [13]. The 28S-D3 gene was amplified using universal primers, D3A (5'-GAC CCG TCT TGA AAC ACG GA-3') and D3B (5'-TCG GAA GGA ACC AGC TAC TA-3'), designed by Litvaitis et al. [14] and later used by Singh et al. [11] and Chen et al. [9]. The PCR reaction conditions were as recommended by Singh et al. [11], except for the amount of primers, which were reduced to 20 pM in 25 µl reactions. Also, the ITS2 region of 23 individuals from different regions, including Minab in Hormozgan Province, was sequenced using the primers and PCR conditions outlined by Manonmani et al. [7].
PCR products were purified using a gel band purification kit (Pharmacia, Piscataway, NJ, USA) according to manufacturer's recommendations and later sequenced at SeqLab laboratory in Germany. The sequence data for the 28S-D3 gene were submitted to Genbank with the accession numbers EU334359-EU334364.
RESULTS
The amplified sequence products with universal primers D3A and D3B were 333 to 338 nucleotides in length. The sequences were edited manually and aligned using the multiple sequence alignment program BioEdit 5.0.6 [15]. All the sequences were homologous except for 1 nucleotide base at position 92 (according to our numbering in Fig. 1, position 84) of sequencing data for species S, T, and U reported by Singh et al. [11].

Alignment of the 28S-D3 sequences for members of the Anopheles fluviatilis species complex and An. fluviatilis specimens from Iran. AF437880, AF437881, and AF437882 are species S, T, and U from India. DQ238495 is the sequence reported by Chen et al. [9] from Iran. EU334359 to EU433364 are related to the present study. EU334359 and EU334361 are from Fars, EU334360 is from Kerman, EU334362 is from Sistan Va Baluchestan, and EU 334363 and EU 334364 are from Hormozgan.
Comparison of the 28S-D3 sequences with other sequences available in Genbank database (Fig. 1) showed that all the specimens from Kazeroun and Khesht regions in Fars Province had the nucleotide base adenine (A) at the position that identifies An. fluviatilis U (Fig. 2A) and were identical with the entries AF-437882 and AJ512735 representing species U in India [9,11]. The individuals from other regions in Hormozgan, Kerman, and Sistan va Baluchestan Provinces were also homologous, but all clearly showed heterozygosity at the position (shown by R in Fig. 2B), which identifies species T and U.
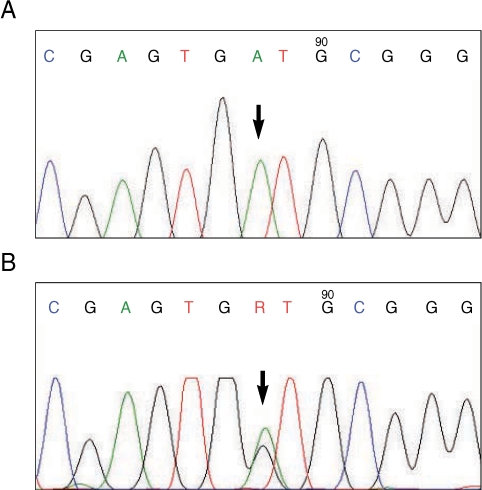
DNA sequence trace data for the 28S-D3 region of An. fluviatilis mosquitoes from Iran. (A) Nucleotide base adenine identifies species U. (B) The code R shows heterozygosity (adenine and guanine) at the position that identifies species U and T.
The ITS2 sequence of all individuals was 374 nucleotides in length, free of ambiguities, and identical to all database entries for species T submitted by other authors [5,7,8].
DISCUSSION
Cytotaxonomic studies in India showed that An. fluviatilis is a complex of 3 reproductively isolated cryptic species, known as S, T, and U [6]. Species S is found to be highly anthropophilic (91%) and is considered the principal malaria vector in India, while species T and U are generally zoophilic (91%) and are regarded as secondary (in the presence of large populations) or non-vectors [16,17]. Manonmani et al. [7] developed an allele-specific PCR assay for the differentiation of the 2 species, referred to as species X and Y, by exploiting differences in the ITS2 gene of rDNA of individuals from Orissa State, India. Subsequent studies showed that cytotaxonomy and ITS2-based methods were 93% concordant for species S and X, and 84% for species T and Y, while no ITS2 gene fragment for species U was reported [18]. The ITS2 sequence of Iranian specimens and species Y (as species T) in India were found to be 100% identical to species T in India [5,8]. Further chromosomal studies on Iranian specimens from Iranshahr showed that they belong to species T [5]. Since ITS2 sequences for An. fluviatilis in all the studied areas, including Iranshahr, were identical, it was concluded that the only prevalent sibling species in Iran was T. Species T is a poor vector in India, while in Iran, it plays an important role in the transmission and maintenance of malaria within its distribution [1,4].
Recently, in laboratory studies, species T was noted to be highly susceptible to sporogony, which potentially makes it an efficient vector when man: cattle ratios are high [19]. A field sporozoite rate as high as 11% in Behbahan, a malaria high risk region in Khozestan Province of Iran during the malaria epidemic of December 1957 [1], might explain the importance of species T in the sustainment and transmission of malaria in the area. Recently, by exploiting the variation in the D3 domain of the 28S rDNA gene, 3 sibling species of S, T, and U were identified [11]. With the emergence of additional rDNA data of the An. minimus and An. fluviatilis complexes, An. fluviatilis species S was recognized conspecific with An. minimus species C (senior synonym) on the basis of homology in the 335 base pair nucleotide sequence of the D3 domain of the 28S gene [9,20]. However, this idea was refuted by Singh et al. [21] who showed that An. fluviatilis S is distinct from An. minimus C based on appreciable differences in the sequences of the ITS2 locus and the 28S D2-D3 domain.
In Iran, screening studies using random amplified polymorphic DNA (RAPD)-PCR showed that the Fars population was different from other sampled populations [8]. Our sequencing data of the 28S-D3 region confirmed the RAPD-PCR results and demonstrated the presence of 2 haplotypes in An. fluviatilis populations in Iran. In Fars Province, all the individuals were identical to species U in India, while in other areas, including Hormozgan, Kerman, and Sistan va Baluchestan, the sequences demonstrated heterozygosity at the position that identifies species T and U in India (Table 1). These 2 species are sympatric in northern India, but exhibit total reproductive isolation as evidenced by the absence of inversion heterozygotes [6,22]. It might be argued that species T and U may occur together in some areas of Iran and hybridize, but heterozygosity is not reflected in the ITS2 region. The high prevalence of heterozygosity in 28S-D3 region cannot be attributed to heterozygote superiority effect, since not a single individual out of all 34 specimens assayed was homozygous for the 28-D3 gene in areas where heterozygosity was prevalent. Such a phenomenon is more likely due to retention of an ancestral polymorphism at the D3 position. Based on 2 references [9,23], Manguin et al. [24] erroneously reported the occurrence of species U in Iran, and mapped its distribution. While the presence of species U is not reflected in the Raeisi et al. [23] and Chen et al. [9] reports, heterozygosity is documented at the position that identifies species T and U [9]. Our findings for the 28S-D3 region in Hormozgan, Sistan va Baluchestan, and Kerman is concordant with those of Chen et al. [9] for 3 Iranian specimens, except for a single nucleotide base at position 309 (Fig. 1). However, in Fars, the only prevalent genotype was identical to the database entries for species U from Uttar Pradesh, India (accession numbers AF437882 and AJ512735) submitted by other authors [9,11]. The 28S-D3 and ITS2 sequences of An. fluviatilis T and U differ by 1 bp and 6 bp (including 4 ambiguities), respectively [9], whereas the ITS2 sequence of the individuals from all regions including Fars, in which the 28S-D3 analysis identifies species U, was identical to species T (Table 1; Fig. 3).

Distribution of An. fluviatilis complex in Iran. Based on the 28S-D3 region, U is An. fluviatilis U, and R is the code for heterozygosity at the nucleotide position that identifies An. fluviatilis U and T. Based on ITS2 region, T is An. fluviatilis T.
The report of another ITS2 variant from Minab in Hormozgan Province (Djadid et al., unpublished data) added to the confusion over the taxonomic status of An. fluviatilis in Iran. It is noteworthy that the ITS2 region of 14 individuals [5,8,present study] from the same region were all identical to database entries for species T from India and Iran. Since An. fluviatilis is believed to be a newly diverged species, it is not expected to observe a high genetic variation within the sibling species complex. The homogeneity of all ITS2 sequences and the presence of only a single nucleotide difference in the 28-D3 region in Iranian specimens cast doubts on the occurrence of An. fluviailis form V in Iran with a variation as high as 1.87% in the ITS2 region. The present study also shows that a single molecular marker is not sufficient to discriminate the taxa over a vast geographical area, and the addition of other molecular markers provides more comprehensive and reliable information.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of our late colleague, Mr. M. Ghasemi, from the Institute of Health Research, Tehran University of Medical sciences, for supplying us with mosquito specimens. This study was supported by a grant from the Pasteur Institute of Iran (No. 292).
