Reactivity of German Cockroach Allergen, Bla g 2, Peptide Fragments to IgE Antibodies in Patients' Sera
Article information
Abstract
Bla g 2 is a cockroach allergen of great importance. This study was conducted to identify IgE-binding epitope(s) of Bla g 2 using the recombinant protein technique. Approximately 50% of tested sera showed IgE reactivity to Pichia-expressed Bla g 2 (PrBla g 2) and E. coli-expressed Bla g 2 (ErBla g 2). Only 5.3% of serum samples showed stronger reactivity to PrBla g 2 than ErBla g 2, indicating that serum was reactive to conformational or carbohydrate epitopes. The full-length and 5 peptide fragments of Bla g 2 were produced in E. coli. All fragments showed IgE-binding activity to the cockroach-allergy patients' sera. Specifically, peptide fragments of amino acid residue 1-75 and 146-225 appeared to be important for IgE-binding. The information about the IgE-binding epitope of Bla g 2 can aid in the diagnosis and treatment for cockroach allergies.
INTRODUCTION
Cockroaches produce allergenic proteins that bind to IgE antibodies and play major roles in allergic diseases. Several allergens from Blattella germanica, or the German cockroach, have been described to date [1]. In a recent study, sensitization to cockroach allergens (Bla g 1, Bla g 2, Bla g 4, Bla g 5, and Per a 7) was assessed in 118 patients with known cockroach allergies [2]. The prevalence of IgE antibodies was highest for the recombinant Bla g 2 (54.4%) among 5 tested allergens [2]. Bla g 2, a glycoprotein with a molecular weight of 36 kDa, has high homology with aspartic proteinases, both in amino acid sequence and in 3-dimensional structure. However, it has not been shown to exhibit enzyme activity [3,4]. Recent studies on the 3-dimensional structure of the Bla g 2 protein revealed that 5 disulfide bridges and a Zn-binding site contribute to its stability [5]. Bla g 2 exists in high concentrations in cockroach digestive organs, such as the esophagus and viscera [6]. Amount of Bla g 2 in B. germanica feces was determined 3 times more than that in the body by 2-site ELISA [7]. However, biological function of Bla g 2 and its mechanism as an allergen remain uncertain.
Various pharmacological approaches can alleviate allergic symptoms; however, only allergen-specific immunotherapy is expected to have a continuous curative effect [8]. High quality cockroach extract is necessary for precise diagnosis and treatment of allergic diseases. However, even high quality extracts may contain allergens to which subjects are not sensitized and may also contain proteolytic enzymes. Proteolytic enzymes can affect the stability of allergens and result in inflammatory responses in subjects [9,10]. Commercially-available cockroach extracts are particularly inconsistent in relative potency and Bla g 2 levels [11]. These difficulties can be minimized through the use of recombinant allergens [12]. B- and T-cell epitopes should be analyzed to attempt precise immunotherapy using recombinant allergens. Allergens with low IgE binding capacities can be synthesized for immunotherapy through substitution of amino acids from epitope regions once an IgE binding epitope is identified [13]. Although Bla g 2 is an important cockroach allergen, research on Bla g 2 B- and T-cell epitopes has not been performed. Recently, B cell epitope was indirectly investigated using mouse monoclonal anti-Bla g 2 antibody inhibiting human IgE binding [14].
The present study was conducted to determine the location of IgE binding epitopes of Bla g 2 through the use of recombinant proteins, and may be helpful for diagnosis and development of novel therapeutic approaches.
MATERIALS AND METHODS
Subjects and sera samples
Patients with asthma, urticaria, rhinitis, or atopic dermatitis seen at the Allergy Clinic of Severance Hospital from 1998 to 2005 were identified, and 38 of these patients with IgE antibodies to B. germanica over 0.7 kU using the Uni-CAP system (Pharmacia, Uppsala, Sweden) were selected (aged 7-65 yr; mean 33 yr). Sera from 20 patients who tested negative by Uni-CAP were used as negative controls.
Expression and purification of full-length and fragmented Bla g 2
A cDNA clone encoding the major Bla g 2 variant (GenBank accession No. EF203068) was used in this study [15]. cDNA encoding full-length Bla g 2 was ligated with the pGEM-T Easy vector (Promega, Madison, Wisconsin, USA) and subcloned into the BamH I and Xho I sites of the pET 28b expression vector. Pichia-expressed Bla g 2 was purchased from INDOOR Biotechnologies Inc. (Manchester, UK) as a control.
The Bla g 2 antigen was divided into 5 fragments containing 5 overlapping amino acids: fragment A (residues 1 to 75), fragment B (residues 71 to 150), fragment C (residues 146 to 225), fragment D (residues 221 to 300), and fragment E (residues 296 to 352) (Fig. 1A). The primer sequences used in the PCR are listed in Table 1. Restriction enzyme sites (BamH I for forward primers and Xho I for reverse primers) were incorporated into each primer sequence for subcloning into the expression vector. Each cDNA fragment was amplified by PCR, ligated initially into the pGEM-T Easy vector, and finally into the pET 28b vector after restriction digestion. Full-length and 5 fragmented recombinant proteins were expressed in E. coli (DE3) and purified by Ni-NTA agarose (Qiagen, Valencia, California, USA) affinity chromatography.
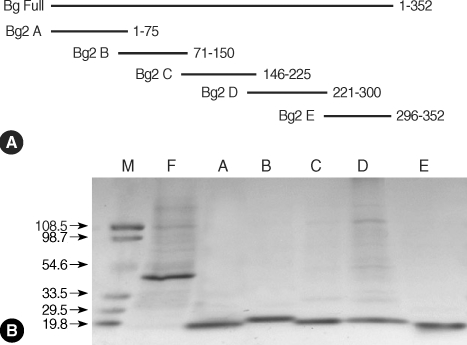
Recombinant ErBla g 2 fragments. (A) Schematic presentation of Bla g 2 fragments for epitope analysis. (B) Purification of full-length and fragments of recombinant Bla g 2. Proteins were separated on a 5-20% gradient SDS-polyacrylamide gel and stained with Coomassie brilliant blue. Lanes: M, molecular mass marker; F, Bla g 2 in full-length; A-E, Bla g 2 fragments A-E. Numbers on left are molecular weight in kDa.
IgE binding reactivity and IgE epitope analysis of recombinant Bla g 2
Reactivity of IgE antibodies to PrBla g 2 and ErBla g 2 was examined by ELISA. Serum samples that displayed reactivity to PrBla g 2 and ErBla g 2 (n = 10), were selected from the initial samples to analyze linear IgE binding epitopes of Bla g 2. In addition, IgE reactivity to Bla g 2 fragments was investigated. Briefly, recombinant proteins (2 µg/ml) were coated (0.1 M sodium carbonate, pH 9.6) onto the microtiter plate (COSTAR, New York, USA). After blocking with 3% skim milk in PBS-0.05% Tween 20 (PBST), the plates were incubated for 1 hr with sample sera (1 : 4 dilution) and PBST containing 1% bovine serum albumin (BSA). IgE antibodies were detected by using biotinylated goat anti-human IgE (1 : 1,000 dilution in PBST containing 1% BSA) (epsilon chain specific) (Vector, Burlingame, California, USA) and streptavidin-peroxidase (1 : 1,000 dilution in PBST containing 1% BSA) (Sigma, St. Louis, Missouri, USA). Optical density at 450 nm was measured after color development by adding 3,3',5,5;-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA) and 1% H2SO4. The mean absorbance level of 15 serum samples, plus 2 standard deviations for the negative control, was used as the cut-off value.
RESULTS
Expression of recombinant Bla g 2 and peptide fragments
The molecular weight of Bla g 2 is estimated to be 36 kDa. However, ErBla g 2 is expressed as a fusion protein and contains an additional 34 amino acids (MGSSHHHHHHSSGLVPRGSHMASMTGGQQMGRDP) at the N-terminus. Recombinant Bla g 2 fragments (8 kDa for fragments A, B, C, and D, and 6 kDa for fragment E) contained an additional 8 amino acids (LEHHHHHH) at the C-terminus, as well as 34 additional amino acids at the N-terminus. Consequently, the molecular masses of the recombinant proteins are 42 kDa for ErBla g 2, 13 kDa for fragments A, B, C, and D, and 11 kDa for fragment E. Molecular masses were determined on a 5-20% gradient SDS-PAGE gel (Fig. 1B).
Comparison of IgE reactivity of PrBla g 2 and ErBla g 2 by ELISA
IgE reactivity to both PrBla g 2 and ErBla g 2 was seen in 50% of serum samples (19 of 38). These subjects were sensitized to cockroach antigens. Only 2 serum samples (5.2%) showed much stronger reactivity to PrBla g 2 than ErBla g 2 (Fig. 2). Samples displaying moderate IgE reactivity to ErBla g 2 (n = 10) were selected for further epitope analysis.
Determination of the IgE epitope
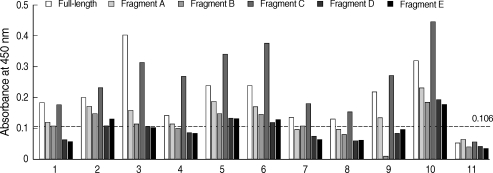
Serum IgE reactivity was noted in varying degrees to different fragments: 8 samples (80%) showed IgE binding reactivity to fragment A, 5 samples (50%) to fragment B, 10 samples (100%) to fragment C, 3 samples (30%) to fragment D, and 4 samples (40%) to fragment E (Fig. 3).
DISCUSSION
Bla g 2 is an important cockroach allergen. Despite this, its biological function and the mechanism by which allergic reactions are elicited to genetically-sensitized individuals have not been elucidated. Recently, recombinant Bla g 2 was produced using an eukaryotic expression system, and its 3-dimensional structure was determined [4,5]. Bla g 2 was found to be inactive and highly stable, and these factors may contribute to sensitization, even at lower levels of exposure.
In the present study, IgE reactivity between PrBla g 2 and ErBla g 2 was compared by ELISA. A minority of samples (2 of 38 positives, 5.2%) showed higher reactivity to PrBla g 2 than ErBla g 2, implying that conformational or carbohydrate epitopes influenced the strong IgE reactivity. However, it is unlikely that carbohydrate epitopes affected IgE reactivity because Bla g 2 was identified by immunoscreening a cDNA bacterial expression library [6]. Most of the serum samples (36 of 38 positives, 94.7%) could not distinguish PrBla g 2 and ErBla g 2, indicating that linear epitopes also contribute to IgE responses. Unexpectedly, Bla g 2 did not elicit strong IgE responses in the Korean population. It is possible that unidentified allergens from cockroaches may play important roles in allergic disease in Koreans [16]. A recent American study in an inner city population suggested that no single allergen was immunologically dominant, and the presence of anti-Bla g 5 correlated with skin test responses in the subjects [17].
It is relatively easy to disrupt conformational epitopes in the production of hypoallergens for allergen-specific immunotherapy. In a recent study, epitope regions of PrBla g 2 were examined by using monoclonal anti-Bla g 2 antibodies which are known to share the binding epitope with human IgE [14]. Both conformational epitope and linear epitopes were described to contribute to the antibody binding. Residues 60-70, 83-86, 98-100, and 129-132 were identified the most important amino acids for the antibody recognition. However, these results are generally not in accordance with the present results, which showed Bla g 2 fragments consisting of residues 146-225, were found to have the strongest IgE binding epitope regions using human serum samples. The results imply the presence of various epitope regions and heterogeneous patterns of IgE reactivity. Specifically, amino acid residues 1-75 and 146-225 included important IgE binding epitope regions. We attempted to analyze linear IgE binding epitopes using synthetic peptides, but could not do so without additional serum samples. Further studies on B- and T-cell epitopes are necessary, and such studies on IgE binding epitopes of Bla g 2 could provide helpful data for the precise diagnosis and treatment of allergic diseases.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Government (MOST) (No. R01-2006-000-10997-0).