Expression of TNF-α and IL-1β in Splenic Dendritic Cells and Their Serum Levels in Mouse Sparganosis
Article information
Abstract
Sparganosis is a tissue invading helminthiasis infecting intermediate hosts, including humans. Strong immune responses are expected to occur in early phases of infection. Thus, we investigated cytokine expressions in splenic dendritic cells and in sera after experimental infection of mice. In splenic dendritic cells, TNF-α and IL-1β expression peaked at week 1 and week 3 post-infection (PI), respectively, and also early phase (week 2 PI) depressed cytokine expression was noticed. Serum IL-1β concentration increased significantly at week 2 PI and peaked at week 6 PI, and that of TNF-α peaked at week 6 PI. These results showed that pro-inflammatory cytokines, TNF-α and IL-1β, are chronologically regulated in mouse sparganosis.
After oral infection, the sparganum (the plerocercoid of Spirometra mansoni) penetrates the intestinal wall and migrates to the peritoneal cavity within a few hours. Finally, worms move to subcutaneous tissues and form a subcutaneous mass in the skin [1].
Dendritic cells (DC) are known as the most potent antigen-presenting cells due to their ability of inducing primary immune responses and activating immune memory cells [2]. Immature DC in the peripheral tissues capture antigens and have the unique migrating capacity to the T cell areas of secondary lymphoid organs. As the DC migrate, DC alter their expression profiles of cell surface molecules, including chemokine receptors and finally activate the resting T cells in the lymph nodes [3,4].
Pro-inflammatory cytokines, such as TNF-α and IL-1β, are pleiotropic cytokines with the host defense inflammatory responses [5,6]. Also, the induction and regulation of inflammatory responses has been shown to be under the control of key pro-inflammatory cytokines, including TNF-α and IL-1β [7] which are produced predominantly by antigen presenting cells, including macrophages. After then, numerous inflammatory mediators should serve to amplify and regulate innate immunity [8]. In tissue invading parasitic infections in immunodeficient mice, for example, with a filaria Brugia sp., pro-inflammatory cytokines, such as TNF-α and IL-1, are known to produce locally in parasitized lymphatics [9]. Therefore, in the present experiment, the author detected cytokine expressions in splenic dendritic cells and sera in order to clarify cellular immune responses in sparganosis of mice.
Spargana were collected from subcutaneous tissues of naturally infected snakes (Rhabdophis tigrina). After washing with sterile physiologic saline, worms were stored at 4℃ until infecton to BALB/c mice. Mice were divided into normal and experimental groups (n=3 for each group), from day 3 to week 8 post-infection (PI). Each mouse was infected by a single sparganum. After sacrificing mice, sera were collected and stored at -70℃ until cytokine assays. The spleen cells were digested with collagenase/EDTA solution. A positive magnetic bead using the mouse dendritic cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) was reacted, and splenic DC were collected by passing over a Midi column (Miltenyi Biotec). RNA was prepared using RNA easy columns (Intron, Seoul, Korea). Primer sets of TNF-α and IL-1β were purchased from Bioneer (Cheongwon, Korea). Their primer information is listed in Table 1. All procedures were performed according to the manufacturer's instructions. Briefly, RT-PCR reactions using the 1-step RT premix (Intron) were performed with an annealing temperature of 55℃ for 35 cycles. Expression of cytokines was calculated with reference to the expression of β-actin and determined in duplicate. Serum cytokine levels were assayed using the mouse IL-1β EIA kit (IBL, Gunma, Japan), mouse TNF-α ELISA kit (Diaclone Research, Besancon, France) and measured duplicate. Results were expressed as a mean (ng/ml)±SD concentration. Statistical significance was analyzed by the Student's t-test (P<0.05).
As shown in Fig. 1, the level of TNF-α increased rapidly and significantly and peaked at week 1 PI and that of IL-1β peaked at week 3 PI. However, TNF-α and IL-1β expressions showed a decreasing tendency at week 2 PI. In addition, serum TNF-α did not increase after infection and serum IL-1β increased slightly at week 2 PI and peaked at week 6 PI (Fig. 2). In this experiment, IL-1β appeared more important than TNF-α in host immune responses against mouse sparganosis.
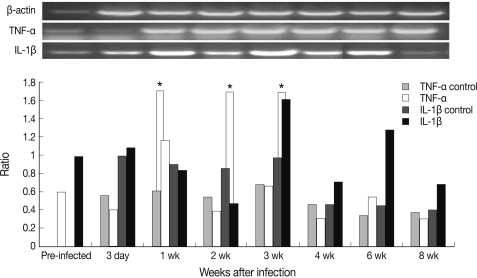
Chronological cytokine expression in splenic dendritic cells in mouse sparganosis. Note that the expression of TNF-α and IL-1β peaked at week 1 and week 3 post-infection, respectively. *P<0.05.

Serum concentration of TNF-α and IL-1β in mouse sparganosis. Note that the serum concentration of IL-1β became significantly higher at week 2 and peaked at week 6 after infection. *P<0.05.
There have been very few reports in which tissue invading cestodiasis, including sparganum, was investigated whether DC have critical protective roles during the infection. Therefore, the present study was to investigate the roles of splenic DC and the levels of early phase pro-inflammatory cytokines, such as TNF-α and IL-1β. Secretions of IL-1β and TNF-α may cause a systemic and a local inflammation. IL-1β has a capacity to downregulate Th2 type responses [10]. The late increase of IL-1β may have induced the development of dominant Th2 cytokine responses, and this may have comprised an early escape mechanism allowing survival and growth of spargana in the host. In another report in which pheripheral blood mono-nuclear cells were stimulated by the Echinococcus multilocularis vesicular antigen, secreted IL-1β and IL-18 levels were reduced [11]. Furthermore, IL-1β promotes cytokine expressions of TNF-α, IFN-γ, and GM-CSF, which results in enhancing the effector cellular cytotoxicity showing differential cytokine expressions [11].
This selective and differential expression of pro-inflammatory cytokines may contribute to the host immune responses for attraction of effector cells to nearby sites of the parasites with the capacity to limit the migration of tissue invading parasites, including spargana. On the contrary, the TNF-α response was preceeded by the IL-1β response in the present study and this means that TNF-α may send signals to provoke IL-1β production in late phases of infection from immune cells. It is certain that the sparganum promote pro-inflammatory cytokine production and splenic DC innate immune responses are an essential first-line defense against the invading sparganum. In this study, serum TNF-α and IL-1β were reduced (P<0.05) in early phases of infection when compared with normal control mouse sera. Such diminished production of IL-1β of immune cells is difficult to explain. Several reports [12,13] suggested that helminth parasite and their secreted products could modulate and suppress DC functions. An explanation have been issued by the presence of viable E. multilocularis metacestodes and possibly mediated by parasite's excretory-secretory molecules that depressed the cytokine release and finally these molecules may prevent inflammatory reactions [11].
In conclusion, the current study provided evidence that the types and magnitudes of TNF-α and IL-1β responses in splenic DC and serum are chronologically regulated during the course of mouse sparganosis. It is suggested that certain pro-inflammatory cytokines may play an important role in the pathogenesis of mouse sparganosis.
ACKNOWLEDGEMENTS
The author thanks Mr. Je-Young Ryu for his laboratory technical assistances. This research was supported by grants from the National Institute of Health (2003-0500-1-1), Ministry of Health and Welfare, Republic of Korea.
