NF-κB and CREB Are Involved in IL-8 Production of Human Neutrophils Induced by Trichomonas vaginalis-Derived Secretory Products
Article information
Abstract
Trichomonas vaginalis is a flagellated lumen-dwelling extracellular protozoan parasite that causes human trichomoniasis via sexual intercourse. Human neutrophils play a crucial role in acute tissue inflammatory responses in T. vaginalis infection. In this study, we investigated the signaling mechanism of neutrophil responses when stimulated with T. vaginalis-derived secretory products (TvSP), which were collected from 1×107 live trichomonads. Incubation of human neutrophils isolated from peripheral blood with TvSP induced up-regulation of IL-8 protein secretion. In addition, stimulation with TvSP induced phosphorylation of NF-κB and CREB in neutrophils. Moreover, TvSP-induced IL-8 production was also significantly inhibited by pretreatment of neutrophils with iκB inhibitor or CREB inhibitor. These results suggest that transcription factors NF-κB and CREB are involved in IL-8 production in human neutrophils induced by stimulation with T. vaginalis infection.
Trichomonas vaginalis is a flagellated protozoan parasite that causes vaginitis and cervicitis in women and asymptomatic urethritis and prostatitis in men, respectively [1,2]. Human infection is mainly transmitted by sexual intercourse [3]. Typical symptoms in the infected women show a purulent and frothy vaginal discharge and develop an itching sensation [4]. During the infection, human neutrophils have been reported to be a major player to control tissue inflammatory responses in response to infections with T. vaginalis [1]. For example, a large number of human neutrophils have been found in the vaginal discharge of women infected with T. vaginalis [5]. In addition, in vitro experiments revealed that the life span of human neutrophils can be prolonged by stimulation with T. vaginalis [6], and that human neutrophils can produce chemokine IL-8 in response to T. vaginalis stimulation [7]. However, information regarding the signaling mechanisms of neutrophil responses induced by T. vaginalis infection are not fully understood. In particular, it has been previously shown that NF-κB or CREB is critical for CXC chemokine gene expression [8]. In this study, we therefore examined inhibitory effects of iκB inhibitor (Bay 11-7082), which inhibits NF-κB phosphorylation, or cAMP-dependent protein kinase (PKA) inhibitor H-89 dihydrochloride which inhibits cAMP response element-binding (CREB) phosphorylation, on T. vaginalis-induced IL-8 production in human neutrophils.
H-89 dihydrochloride was purchased from Calbiochem (Gibbstown, New Jersey, USA), and Bay 11-7082 was purchased from Enzo Life Sciences (Farmingdale, New York, USA). Mouse anti-human CD16 mAb conjugated with magnetic particle was purchased from MACS (Miltenyi Biotec, Bergisch Gladbach, Germany). All other reagents were purchased from Sigma Chemical Company (St. Louis, Missouri, USA). T016 strain of T. vaginalis was used in this experiment. T. vaginalis was axenically subcultivated at 37℃ with Diamond's trypticase-yeast extract-maltose (TYM) medium with 10% heat-inactivated horse serum (Gibco/Invitrogen, Gaithersburg, Maryland, USA) and 0.5% penicillin/streptomycin (Gibco/Invitrogen). For preparation of T. vaginalis-derived secretory products (TvSP), trichomonads were obtained from the logarithmic phase of growth. Trichomonads (1×107) were resuspended in 1 ml of HBSS, and incubated for 1 hr at 37℃ in order to obtain TvSP. After incubation, culture supernatants were centrifuged for 10 min at 10,000 g and filtered through a 0.22 µm pore size filter, yielding TvSP used in this study. Human neutrophils were isolated from peripheral blood of normal volunteers, who had no allergic or infectious diseases, by density gradient centrifugation and positive immunomagnetic selection. Briefly, diluted venous blood (30 ml) was overlayered on a Histopaque (density 1.083 g/ml) and centrifuged at 1,000 g at 4℃ for 30 min. After removal of the supernatant and mononuclear cells at the interface, erythrocytes in the sediments were lysed by exposure to 2 cycles of sterile distilled water. Isolated granulocytes were incubated with mouse anti-human CD16 mAb conjugated with magnetic particles for 30 min on ice and then the cells were loaded on the separation column positioned in the MACS magnetic field. Cells were eluted with 15 ml of PIPES buffer with 1% FCS to remove eosinophils. The purity of neutrophils counted by Randolph's stain was consistently over 99%. Purified neutrophils were used immediately for experiments.
For immunoblot, isolated neutrophils (5×105) were stimulated for various time points with TvSP. After stimulation, whole cell lysates were separated by 7.5% SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane (Millipore, Massachusetts, USA). The membrane was incubated with primary antibodies specific for phospho-CREB (Cell Signaling Technology, Danvers, Massachusetts, USA), phospho-NF-κB (p65) (Cell Signaling Technology), or β-actin (Cell Signaling Technology) for 2 hr at room temperature or overnight at 4℃. The membrane was then incubated with horseradish peroxidase (HRP)-conjugated anti-mouse, anti-rabbit, or anti-goat IgG antibody at RT for 1 hr. Immunoreactivity was detected with LumiGLO (Cell Signaling Technology). Neutrophils (5×105/well) seeded in 24-well tissue culture plates were also pretreated for 30 min with or without Bay-11-7082 or H-89 dihydrochloride, and then incubated for 3 hr with or without 100 µl TvSP collected from 1×107 trichomonads at 37℃ in a 5% CO2 incubator. No cytotoxicity of neutrophils was observed with the inhibitors at the concentrations used in this study (data not shown). After incubation, culture supernatants were collected from neutrophils incubated in various conditions, and analyzed by a specific human IL-8 ELISA set (Thermo Scientific, Waltham, Massachusetts, USA) according to the manufacturer's instructions. Data were expressed as the mean and standard error from 3 independent experiments in duplicate. Statistical significance between treatment and control groups was assessed by the paired Student's t-test. A probability value of less than 0.05 was considered significant.
We first examined whether TvSP can induce activation of NF-κB and CREB in human neutrophils. As shown in Fig. 1, when neutrophils were stimulated for up to 60 min with TvSP, phosphorylation of NF-κB or CREB were increased compared to results for cells incubated with medium alone. For example, phosphorylation status of NF-κB or CREB started to increase after 15 min of stimulation with TvSP. Next, using specific inhibitors, we examined whether NF-κB or CREB is closely involved in IL-8 production in neutrophils induced by TvSP. As shown in Fig. 2A and 2B, when human neutrophils were stimulated for 3 hr with TvSP, IL-8 production was significantly increased compared to results for cells incubated without TvSP. For example, when nutrophils were incubated with medium alone, the amount of IL-8 released was 536.5±16.65 pg/ml. However, neutrophils were incubated with TvSP, IL-8 production was significantly increased to 968.4±36.3 pg/ml. In contrast, TvSP-induced IL-8 production was dose-dependently inhibited by pretreatment of neutrophils with iκB inhibitor Bay 11-7082 and PKA inhibitor H-89 dihydrochloride, respectively. These results suggest that activation of NF-κB and CREB might be important to mediate transcription of IL-8 in human neutrophils induced by TvSP.
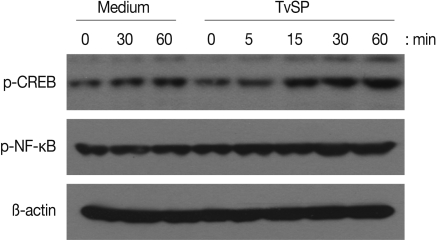
Effects of TvSP on phosphorylation of NF-κB (A) and CREB (B) in human neutrophils. Neutrophils were stimulated for 5, 15, 30 or 60 min with or without TvSP collected from 1×107 trichomonads. After stimulation, whole cell lysates were subjected to SDS-PAGE and immunoblotted with Ab specific for phospho-NF-κB, phospho-CREB, or β-actin as a control.
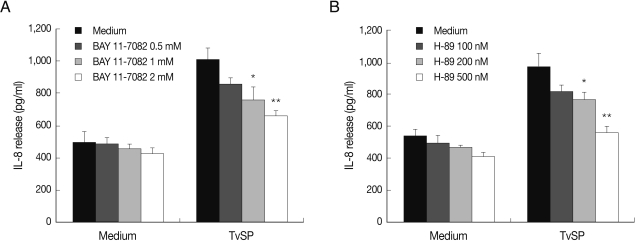
Effects of NF-κB inhibitor (Bay 11-7082) (A) or CREB inhibitor (H89) (B) on TvSP-induced IL-8 production in human neutrophils. Neutrophils (5×105) were pretreated for 30 min with or without Bay 11-7082 or H89 were stimulated for 3 hr with or without TvSP. After stimulation, IL-8 protein in culture supernatants was measured by ELISA. Data are expressed as mean±SEM from 3 independent experiments. Significant differences from the value obtained with cells incubated in medium alone are shown. *P<0.05; **P<0.01.
Although there has been a previous report that T. vaginalis induces NF-κB activation in macrophages [9] leading to production of proinflammatory cytokines, we showed for the first time that CREB as well as NF-κB might be involved in IL-8 production in human neutrophils induced by T. vaginalis. Several papers have shown that various immune cells can produce IL-8 in response to various components of T. vaginalis. For instance, the membrane components of T. vaginalis stimulate human monocytes to release IL-8 [10], and trichomonad surface glycoconjugate lipophosphoglycan (LPG) induces IL-8 production in vaginal epithelial cells via a TLR-independent mechanism [11]. Moreover, it has recently been demonstrated that T. vaginalis-secreted protein AP65 triggers production of IL-8 in human vaginal epithelial cells [12].
Although TvSP collected from live trichomonads has a capability to activate human neutrophils leading to IL-8 production [7], information regarding the specific secretory component responsible for activation of human neutrophils is limited. Interestingly, a previous report has shown that T. vaginalis can secrete a kind of lipid mediators, leukotriene B4 (LTB4), which can induce activation of human neutrophils resulting in migration [13]. It is generally accepted that human LTB4 has been shown to activate immune cells to produce chemokines through activation of NF-κB. For example, LTB4 stimulates human monocytes to secrete MCP-1 via NF-κB activation [14]. Very recently, we have demonstrated that LTB4 receptor BLT1- and BLT2-mediated activation of NF-κB and CREB are closely involved in IL-8 production of human mast cells induced by TvSP [15]. These results led us to speculate a hypothesis that T. vaginalis-secreted LTB4 may be a stimulus for activation of transcription factors NF-κB and CREB required for IL-8 production in human neutrophils stimulated with TvSP. Therefore, further studies to elucidate the signaling role of LTB4 secreted from T. vaginalis in neutrophil activation are needed.
In conclusion, we have demonstrated that TvSP contributes to IL-8 production in human neutrophils via activation of the transcription factors NF-κB and CREB in the context of T. vaginalis infection. Deeper excavation on the crosstalk between human neutrophils and T. vaginalis may elucidate the uncovered secret regarding delicate balance between the host and parasite in the affected tissues during human trichomoniasis, particularly in women.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-314-E00070).