Inhibitory Effects of Iranian Thymus vulgaris Extracts on in Vitro Growth of Entamoeba histolytica
Article information
Abstract
One of the most common drugs used against a wide variety of anaerobic protozoan parasites is metronidazole. However, this drug is mutagenic for bacteria and is a potent carcinogen for rodents. Thymus vulgaris is used for cough suppression and relief of dyspepsia. Also it has antibacterial and antifungal properties. The aim of this study was to investigate antiamebic effect of Thymus vulgaris against Entamoeba histolytica in comparison with metronidazole. One hundred gram air-dried T. vulgaris plant was obtained and macerated at 25℃ for 14 days using n-hexane and a mixture of ethanol and water. For essential oil isolation T. vulgaris was subjected to hydrodistillation using a clevenger-type apparatus for 3 hr. E. histolytica, HM-1: IMSS strain was used in all experiments. It was found that the minimal inhibitory concentration (MIC) for T. vulgaris hydroalcoholic, hexanic extracts, and the essential oil after 24 hr was 4 mg/mL, 4 mg/mL, and 0.7 mg/mL, respectively. After 48 hr the MIC for T. vulgaris hydroalcoholic and hexanic extracts was 3 and 3 mg/mL, respectively. Therefore, it can be concluded that the Iranian T. vulgaris is effective against the trophozoites of E. histolytica.
INTRODUCTION
Amebiasis is caused by Entamoeba histolytica, a protozoan parasite that is 10 to 60 µm in length and moves through the extension of finger-like pseudopods [1]. Spread occurs via the fecal-oral route; usually by poor hygiene during food preparation and eating or by the use of ''night soil'' (crop fertilization with human waste), as well as by oral-anal sexual habits. Crowding and poor hygiene contribute to its prevalence in Asia, Africa, and Latin America. Fifty million people suffer from amebic colitis and liver abscess caused by E. histolytica resulting in 50,000 to 100,000 deaths yearly [2,3]. The stable reservoir of infective cases causes difficulties in eradication. After malaria, it is likely that E. histolytica is the world's second leading protozoan cause of death [4]. Among children under 5 yr who were admitted with acute diarrhea in a hospital, E. histolytica was confirmed in 7.8% of the cases [5]. The estimated number of infected cases may be much higher due to asymptomatic diseases and lack of a sensitive and specific diagnostic test [6].
The most effective and commonly used drug for treatment of intestinal protozoan infection is metronidazole. However, this drug has been reported to have unpleasant side effects such as metallic taste, headache, dry mouth, and to a lesser extent nausea, glossitis, urticaria, pruritus, and dark colored urine. In addition, carcinogenic, teratogenic, and embryogenic effects have been documented [7,8]. Thus, the search for alternative antiamebic compounds with high activity, low toxicity, cheaper, and more effective is still a necessary goal.
Thymus vulgaris (garden thyme) is a small shrubby plant with a strong, spicy taste, and odor. This plant is indigenous to the Mediterranean region of Europe and is extensively cultured in the United States. Known primary constituents of the thyme include essential oil (borneol, carvacrol, cymol, linalool, and thymol), tannin, flavonoids (apigenin and luteolin), saponins, and triterpenic acids. Essential oil and extracts from fresh leaves and flowers can be used as aromatic additives in foods, pharmaceuticals, and cosmetics. T. vulgaris has antispasmodic, carminative, diaphoretic, expectorant, and sedative properties. As a tinture, extract, or infusion, thyme is frequently used in throat and bronchial problems, including acute bronchitis, laryngitis, and whooping cough, and also for diarrhea, chronic gastritis, and loss of appetite [9].
The aim of this study was to investigate antiamebic effect of Thymus vulgaris against Entamoeba histolytica in comparison with metronidazole.
MATERIALS AND METHODS
Hydroalcoholic and hexanic extracts preparation
T. vulgaris was supplied by the Tehran University Institute of Medicinal Plants (IMP). One hundred gram of air-dried T. vulgaris was obtained and macerated at 25℃ for 14 days using n-hexane and a mixture of ethanol and water (90 : 10 ratio). The extracts were obtained and filtered using Whatman No.1 filter paper. The filtrates were concentrated with a rotary evaporator at 40℃ to facilitate their further freeze-drying process. The concentrated extracts were finally freeze-dried at -50℃ for 24 hr and stored at -20℃. The extracts were redissolved in their solvents before each individual experiment [10].
The yield of T. vulgaris using ethanol/water (90 : 10) as solvent was 10.55% dry extract and with n-hexane was 3.44% dry extract.
Preparation of essential oil
One hundred gram of air-dried T. vulgaris was subjected to hydrodistillation using a clevenger-type apparatus for 3 hr. The essential oil obtained was dried with anhydrous sodium sulfate and kept at 4℃ [11]. The hydrodistillation of the air-dried plant yielded clear oil. The yield of essential oil obtained by hydrodistillation was 4.33% with a characteristic odor.
Entamoeba histolytica culture
E. histolytica HM-1: IMSS strain was used in all experiments. The trophozoites were cultured axenically in screw-capped tubes at 35.5℃ on Diamond's TYI-S-33 medium, supplemented with 10% (v/v) heat-inactivated bovine serum [12]. Subcultures were performed routinely at 48 hr intervals by replacing the medium without detaching the monolayer. Cells were harvested by replacing the medium with a fresh one, chilling on ice for 20 min, and inverting gently to detach the monolayer.
Preparation of extracts and essential oil stock solutions
One hundred mg/mL of each extract was dissolved in its solvent, sterilized through a 0.22 µm Millipore filter, and kept as stock solution. The essential oil was sterilized through a 0.22 µm Millipore filter and then was emulsified by 5% sterilized Tween 80 to be mixed with culture medium.
In vitro exposure and evaluation
Different concentrations (ranged 0-10 mg/mL) of plant extracts and essential oil were prepared in test tubes by serial dilutions and air-dried under sterile conditions. Diamond's TYI-S-33 medium was added to the test tubes. The tubes contents were then turned into a suspension by using the ultrasound bath for 30 min and were kept at rest for 24 hr at 4℃. Subsequently 1.0 × 103 trophozoites of E. histolytica were added to the test tubes and then incubated at 35.5℃ for 24 hr and 48 hr.
The tests were also repeated using the standard amebicidal drug, i.e., metronidazole. The experimental groups were assigned in the following manner: 1) Diamond's TYI-S-33 medium + trophozoites, 2) Diamond's TYI-S-33 medium + trophozoites + 5% Tween 80, and 3) Diamond's TYI-S-33 medium + trophozoites + metronidazole (0.5, 1, 1.5, and 2 µg/mL).
After the 24 hr and 48 hr periods the tubes were chilled for 20 min and the attached trophozoites were detached by gentle inversion. The number of viable cells was determined using eosin 0.01% [13] and the microscope (the Neubauer chamber). Motility and dye exclusion were the criteria for viability.
The lowest concentration of each plant extract and essential oil which completely inhibited the growth of trophozoites of E. histolytica was considered as MIC [14]. After determining the MIC, concentrations of 1/4, 1/2, and 3/4 of MIC were used.
Growth rate (GR) was defined as the difference between the number of living protozoa counted at 0 hr and after 24 hr and 48 hr. The percentage of growth inhibition (% GI) was calculated using the following formula [15]:
% GI = ( 1 - GRExtract / GRControl ) × 100
The experiments were performed in duplicate and repeated 3 times.
Statistical analysis
Data were analyzed using 2-way analysis of variance (ANOVA) [14].
RESULTS
The inhibitory capacity of the extracts and essential oil of T. vulgaris were asayed using eosin, as a result of which viable trophozoites of E. histolytica remained clear whereas dead cells were light red in color [13]. Incubation of E. histolytica trophozoites in the concentration range 0-10 mg/mL of extracts and essential oil caused growth inhibition. When the concentration range increased, the number of living trophozoites decreased. However, population densities of the amoeba in the control tubes were increased ten times on the average. The percent of GI (growth inhibition) after 24 and 48 hr are shown in the Table 1.

Effect of metronidazole, the hydroalcoholic, hexanic extracts and essential oil of T. vulgaris on trophozoites of E. histolytica after 24 hr and 48 hr incubation
The essential oil was the most active agent against the trophozoites of E. histolytica. The MIC for essential oil after 24 hr was 0.7 mg/mL, whereas the MIC for metronidazole, the current drug of choice, was 2 µg/mL and 1.5 µg/mL after 24 hr and 48 hr, respectively. Hydroalcoholic and hexanic extracts were able to destroy the trophozoites of E. histolytica, but at a higher concentration (4 mg/mL and 3 mg/mL, after 24 hr and 48 hr, respectively) (Figs. 1, 2).
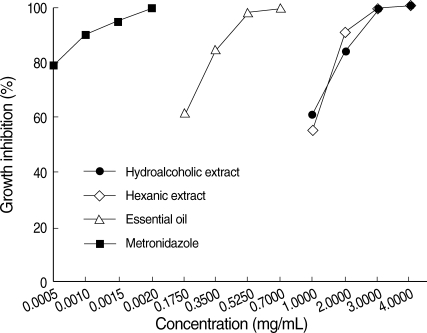
Effects of metronidazole, hydroalcoholic and hexanic extracts, and the essential oil of T. vulgaris on growth inhibition of E. histolytica HM-1: IMSS trophozoites. The percentage of dead trophozoites after their exposure for 24 hr to metronidazole, the extracts, and the essential oil was determined using eosin.
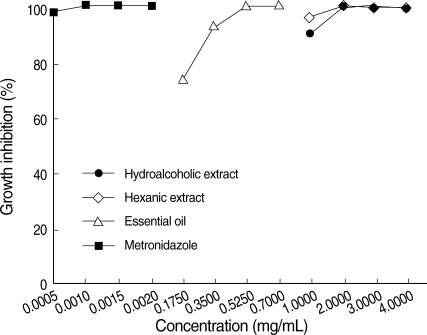
Effects of metronidazole, hydroalcoholic and hexanic extracts, and the essential oil of T. vulgaris on growth inhibition of E. histolytica HM-1: IMSS trophozoites. The percentage of dead trophozoites after their exposure for 48 hr to metronidazole, the extracts, and the essential oil was determined using eosin.
The inhibition appeared to be dose-dependent. Moreover, the results revealed that a longer period of exposure to T. vulgaris at the same concentration decreases the number of viable trophozoites.
DISCUSSION
This study investigated the antiamebic activity of T. vulgaris extracts and essential oil and compared the MICs of these agents with metronidazole. Parasite growth depends on the primary number of active parasites, temperature, and culture medium. In our study, in similar experimental conditions, the results of experimental groups were significantly different from those of the control group (P < 0.01).
The thyme extracts have direct antimicrobial actions and also potentiate the effectiveness of tetracycline against drug-resistant Staphylococcus aureus [16]. Essential oil from T. vulgaris had antiprotozoal activity on the viability of bloodstream forms of Trypanosoma brucei [17] and Trypanosoma cruzi [18]. Methanolic extract of T. vulgaris showed moderate activity on E. histolytica and Giardia lamblia [19].
In our study, T. vulgaris inhibited growth of E. histolytica at low concentrations. In particular, the essential oil of T. vulgaris exhibited stronger activity against E. histolytica trophozoites than the extracts. In fact, Lee et al. (2005) previously showed that the essential oil of T. vulgaris has greater antioxidant activity in comparison with its extracts [20]. It seems likely that antiamebic activity of T. vulgaris is related to thymol, carvacrol, borneol, and linalool which are the most abundant compounds in the essential oil of plant [11]. Considering the above findings, T. vulgaris essential oil and extracts seem to be good antiamebic substitutes for metronidazole.
ACKNOWLEDGEMENTS
This work was supported by a grant from Shahid Beheshti University (M.C) (No. 13/12975) Tehran, Iran. The authors would like to thank Mr. M. Kamalinejad from the School of Pharmacy of Shahid Beheshti University (M.C), Dr. P. Aberumand Azar and Mr. K. Larijani from the Science and Research Branch of Islamic Azad University for their help, and also Ms. Sh. Farnia from the School of Public Health, Tehran University of Medical Sciences for her help with providing the parasite culture.