A Case of Oral Myiasis Caused by Lucilia sericata (Diptera: Calliphoridae) in Korea
Article information
Abstract
We report here a case of oral myiasis in the Republic of Korea. The patient was a 37-year-old man with a 30-year history of Becker's muscular dystrophy. He was intubated due to dyspnea 8 days prior to admission to an intensive care unit (ICU). A few hours after the ICU admission, 43 fly larvae were found during suction of the oral cavity. All maggots were identified as the third instars of Lucilia sericata (Diptera: Calliphoridae) by morphology. We discussed on the characteristics of myiasis acquired in Korea, including the infection risk and predisposing factors.
INTRODUCTION
Oral myiasis is regarded as a type of wound myiasis strongly associated with poor oral hygiene. Many other factors can also play a role, including alcoholism, senility, suppurating lesions, gingival diseases, trauma, paralysis or immobility, and mental debility [1-3].
The most common causative agent of myiasis, the dipteran clade Calyptratae, consists of approximately 18,000 described species, which represents approximately 12% of the known dipteran diversity. Four families of the Calyptratae, including Calliphoridae (blowflies), Sarcophagidae (flesh flies), Oestridae (bot flies), and Muscoidea (dung flies), have been implicated in causing most of the specific or facultative myiasis in humans [4].
Among the members of the Calliphoridae, Lucilia species are distributed throughout the world. They are considered important in the field of medical and veterinary science as causative agents of myiasis in humans and animals. Lucilia sericata is commonly called as the green bottle blowfly or sheep strike blowfly. It is known as the predominant pathogenic species in cool temperate habitats such as New Zealand [5].
The present paper reports a case of oral myiasis caused by L. sericata third instars larvae and documents the morphological characters of maggots observed by light and scanning electron microscopes (SEM). We also discuss the characteristics of 6 cases of myiasis acquired in Korea, including the risk of accidental myiasis in patients with predisposing factors.
CASE DESCRIPTION
Secondary to the diagnosis of Becker's muscular dystrophy, the patient (37-year-old man) has been bedbound for 30 years. On the evening of 21 October 2011, he complained of dyspnea and chest pain while at his nursing home. His blood pressure dropped to 60/30 mmHg and his mental status became drowsy. He was transferred to the emergency room (ER) of a university hospital, and emergency treatment was performed, including intubation. After spending 8 days in the ER, the patient was moved to an intensive care unit (ICU) in Busan St. Mary's Medical Center by the request of a guardian. The patient had symptoms, including hematochezia, fever, and chills. Laboratory findings included WBC of 5,200/mm3, hemoglobin (Hb) concentration of 12.5g/dl, and C-reactive protein (CRP) of 159.45 mg/L. Chest CT scans revealed bronchiectasis in the left lung field. There were no specific findings on abdominal CT scans. Several hours after transfer to the ICU, 38 maggots were removed during suction of the oral cavity. Two hours later, 5 more maggots were removed from the oral cavity. A fecal occult blood test was positive, and Hb taken on the next day was less than the normal range (11.6 g/dl). Laryngoscopic examination revealed no additional maggots or wounds in the nasal cavity or laryngopharyngeal space. The patient's condition improved and he returned to the nursing home on 26 December 2011.
For light microscopic observations, the larvae were preserved in 70% ethanol, and then they were cleared in a 10% aqueous solution of KOH. After clearing, the specimens were mounted on slides using glycerin-jelly medium. Additional maggots were preserved in 2% glutaraldehyde for SEM analysis. After washing 3 times with 2% glutaraldehyde, the specimens were dehydrated through a graded alcohol series (50%, 70%, 80%, 90%, 95%, and absolute alcohol), dried with hexamethyldisilazane, coated with gold (JFC-1100E ion sputtering device), and observed with Electron SEM Philips XL-30S (Philips, London, UK) at a 15-kV accelerating voltage. Species identification was done using the keys described by previous workers [6,7] and the Natural History Museum (London, UK) website (http://www.nhm.ac.uk/research-curation/research/projects/myiasis-larvae/key/).
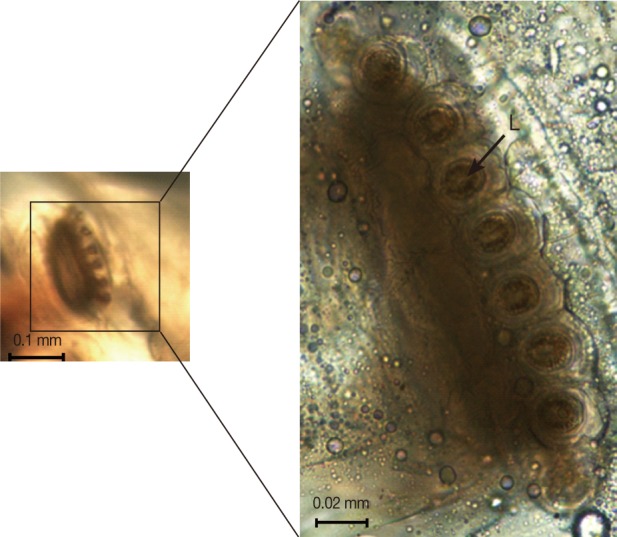
The collected maggots were 9-12 mm (mean; 10.3 mm) in length with a beige coloring (Fig. 1). The larvae showed the typical maggot-like body shape. The pseudocephalon had a pair of blunting antennae, each equipped with 2 sensory papillae. A pair of hooks was also located in the buccal cavity (Fig. 2A). A pigmented accessory oral sclerite was not observed in the cephalopharyngeal skeleton. The anus had 1 pair of anal papillae surrounded by numerous tiny spines and was located under the posterior spiracular plate in the last segment (Fig. 2B). The body surface was covered with many tiny short spines. The body spines were sharp with a single point and arranged in distinct bands around the body showing clearly defined spine bands (Fig. 2C-F). Anterior spiracles showed a fan-like structure with 8 lobes (Fig. 3). The posterior spiracles were not positioned within a cavity (Fig. 4A). The shape of the posterior spiracles was approximately round with a surrounding complete peritremal ring with no gap (Fig. 4B). The button was present on both spiracles, and 3 slits in each posterior spiracle were straight (Fig. 4C). The distances (d1, d2, and d3) of the dorsal papillae (p1), median papillae (p2), and outer papillae (p3) in the 12th segment were similar (Fig. 4D).

Dorsal view of third instars larvae of Lucilia sericata isolated from the patient's oral cavity. The larvae showed the typical maggot-like body shape.
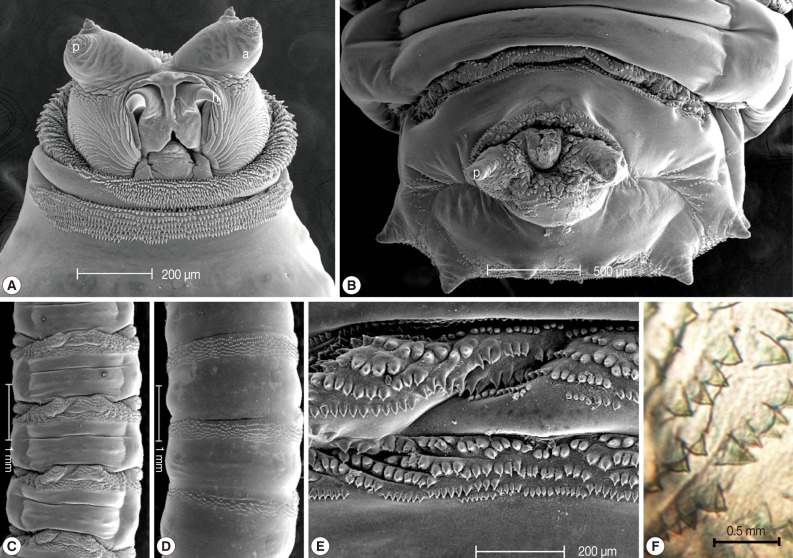
Light microscopic and SEM views of the surface of a maggot. The cephalic segment had 1 pair of antennae with 2 sensory papillae for each, and the buccal cavity had 1 pair of hooks (A). The anus had 1 pair of anal papillae (B). The body was covered with many tiny, short spines (ventral and dorsal side, C and D). The spines were arranged in distinct bands around the body showing clearly defined spine bands (C, D). The spines were sharp with a single point (E, F). a; antenna, p; papilla, h; hook.
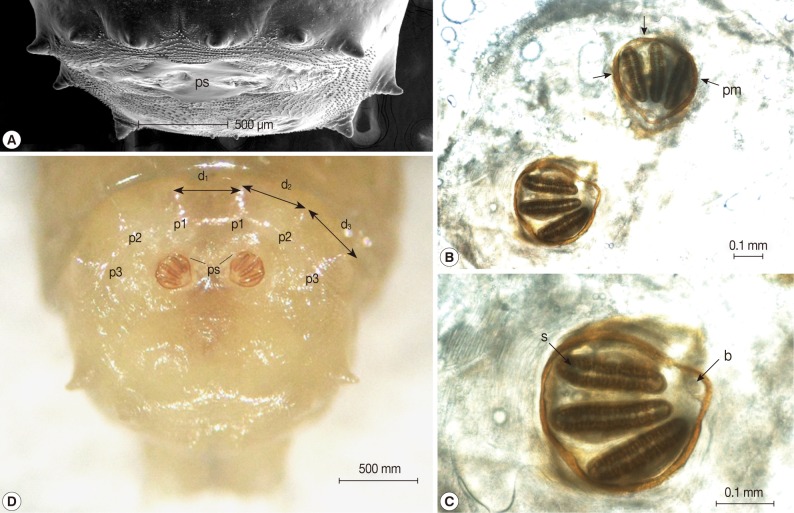
Light microscopic and SEM views of the posterior part of the maggot. (A) The posterior spiracles (ps) were not positioned within a cavity. (B) The shape of the posterior spiracles was approximately round surrounded by a complete peritremal ring (pm) with no gap. (C) The button (b) presented on both spiracles and the 3 slits (s) in each posterior spiracle were straight. (D) The distances (d1, d2, and d3) of the dorsal papillae (p1), median papillae (p2), and outer papillae (p3) in the 12th segment were similar (D).
DISCUSSION
The family Calliphoridae contains more than 1,100 species, which can be found in almost every terrestrial habitat. Most of the species are of medical importance due to their abundance and distribution. The members of the Calliphoridae are also the most common cause of Korea-acquired myiasis. Among 6 reported cases of myiasis, 5 were caused by Lucilia species with the remaining one by the result of a Phormia species infection [8-13].
Myiasis caused by L. sericata can be categorized as accidental myiasis in the ecological classification [14]. Free-living Lucilia larvae can cause a pathological reaction when coming in incidental contact with the host. In an analysis by Sherman [15] of maggots from 20 medical centers in the US, the most common species isolated was L. sericata, which was isolated from 71% (30/42) of the patients [15]. However, when Sherman [15] compared his results to 72 previous reports that described 137 cases of US-acquired human myiasis from1960 to 1995, the results greatly differed. The most common causative species was the rodent botfly (Cuterebra species), followed by the screwworm fly (Cochliomyia hominivorax), and sheep botfly (Oestrus ovis). L. sericata was identified only in 8 cases (5.8%). This discrepancy was assumed to be a result of underreporting of non-invasive myiasis. One reason for this was that non-invasive myiasis was considered unworthy of reporting [15]. Lukin's prospective study [16] in Australia also showed similar results; Lucilia cuprina was the causative agent in 12 out of 14 recorded cases from 1986 to 1988 in Brisbane. L. cuprina is well known as the predominant species in subtropical regions, while L. seretica is dominant in cool temperate areas. The fact that 5 cases of myiasis acquired in Korea were caused by Lucilia species is in line with these reports.
We suspected that the present case was a nosocomial infection. The patient had spent 8 days in the ER, and the maggots were found on the day of admission to the ICU. Based on L. sericata's life cycle, the infection was most likely acquired in the ER [17]. Lukin [16] and Sherman [15] reported 5% and 43% of nosocomial infections in North America and Australia, re-spectively. A review of myiasis acquired in Korea, however, revealed that 4 of 6 cases were nosocomial, or highly suspected to be nosocomial. Flies usually attack patients who are immo-bile, injured, or severely ill, as was the case with the present patient during the summer. Therefore, special care must be taken to secure the hygiene of environments such as the ER and ICU for patients with predisposing factors for myiasis.
In conclusion, although L. sericata is not an obligatory parasite, it has a high probability of causing accidental myiasis. Since myiasis can exacerbate the severity of a disease or be fatal to patients, patients with predisposing factors such as advanced age, debilitating conditions, peripheral vascular diseases, chronic wounds, paralysis or immobility, and mental debility should be carefully protected from flies to prevent accidental and nosocomial myiasis.