A 27 kDa Cysteine Protease Secreted by Newly Excysted Paragonimus westermani Metacercariae Induces Superoxide Anion Production and Degranulation of Human Eosinophils
Article information
Abstract
Eosinophil degranulation plays a crucial role in tissue inflammatory reactions associated with helminth parasitic infections and allergic diseases. Paragonimus westermani, a lung fluke causing human paragonimiasis, secretes a large amount of cysteine proteases, which are involved in nutrient uptake, tissue invasion, and modulation of hos's immune responses. There is, however, limited information about the response of eosinophils to direct stimulation by cysteine proteases (CP) secreted by P. westermani. In the present study, we tested whether degranulation and superoxide production from human eosinophils can be induced by stimulation of the 2 CP (27 kDa and 28 kDa) purified from excretory-secretory products (ESP) of P. westermani newly excysted metacercariae (PwNEM). A large quantity of eosinophil-derived neurotoxin (EDN) was detected in the culture supernatant when human eosinophils isolated from the peripheral blood were incubated with the purified 27 kDa CP. Furthermore, the 27 kDa CP induced superoxide anion production by eosinophils in time- and dose-dependent manners. In contrast, the purified 28 kDa CP did not induce superoxide production and degranulation. These findings suggest that the 27 kDa CP secreted by PwNEM induces superoxide production and degranulation of human eosinophils, which may be involved in eosinophil-mediated tissue inflammatory responses during the larval migration in human paragonimiasis.
Paragonimus westermani is a tissue-invasive helminthic parasite that causes pulmonary or extrapulmonary paragonimiasis in humans. P. westermani newly excysted larvae (PwNEM) secrete excretory-secretory products (ESP) that are mainly composed of 2 cysteine proteases of 27 and 28 kDa size, which are required for excystment of the metacercariae [1], successful larval tissue invasion [2], and evasion of the host's immune system [3]. Moreover, recent studies have shown that proteases in the ESP secreted by PwNEM regulate IL-8 production and the life span of human eosinophils [4,5]. These results led us to speculate potential roles of Paragonimus-secreted cysteine protease (CP) in eosinophil-mediated tissue inflammation in infected lesions of the parasite. Eosinophils play a key role in tissue inflammation during helminth infection and allergic diseases [6]. Release of cytotoxic granule proteins and oxygen radicals from activated eosinophils in inflamed tissues is a key effecter mechanism in the process of tissue inflammation [7]. However, there is limited data regarding the role of 27 kDa or 28 Da CP secreted by PwNEM in the effecter function of human eosinophils. To address this question, we purified 27 and 28 kDa CP from the ESP produced by PwNEM, and then tested the effects of each CP on superoxide anion production and degranulation of human eosinophils.
The ESP of PwNEM was prepared freshly from 5,000 PwNEM isolated from the naturally infected freshwater crayfish (Cambaroides similis) as described in a previous study [8]. A 1.6 × 4 cm long DEAE-trisacryl M anion-exchange column (Pharmacia-LKB, Piscataway, New Jersey, USA), pre-equilibrated with sodium phosphate buffer (0.02 M, pH 6.3), was used to purify each CP from the ESP. A total of 2.5 ml crude ESP was eluted through 0.02, 0.05, 0.1, and 0.2 M NaCl in a stepwise fashion. Active fractions for 27 or 28 kDa CP were collected, dialyzed against distilled water and then aseptically filtered. The purified CP filtrates were lyophilized, diluted with an appropriate medium to the desired concentration and stored at -20℃ before use. The purity of the purified enzymes was analyzed by SDS-PAGE (Fig. 1). The protein amount of the purified CP was measured using the bicinchoninic acid protein assay kit (Pierce, Rockford, Illinois, USA), and the proteolytic activity was determined by the IgG cleavage assay as described previously [3]. Human eosinophils were isolated by the method described previously [9] using immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and the magnetic cell separation system (MACS) (Miltenyi Biotec) from the peripheral blood of healthy individuals. The purity of the eosinophils as measured by Randolph's staining was consistently greater than 95%. In order to measure eosinophil superoxide anion production and degranulation, 100 µl cell suspension (0.5 × 106 cells/ml) in Hanks balanced salt solution (HBSS) (pH 7.4) with 10 mM HEPES, 0.03% gelatin and 100 µM cytochrome c (Sigma, St. Louis, Missouri, USA) were seeded in the wells of 96-well tissue culture plates. The reactions were initiated by adding 100 µl HBSS medium containing 27 kDa CP (0.625, 1.25, 2.5, and 5 µg), 28 kDa CP (0.2, 0.6, 2, and 6 µg), or medium alone. The plate was incubated at 37℃ and the reaction was measured for absorbance at 550 nm in a microplate reader (Thermomax; Molecular Device, Sunnyvale, Caliornia, USA) in various time intervals from 0 to 3 hr. Superoxide anion production was calculated with an extinction coefficient of 21.1 × 10-3 M/cm for reduced cytochrome c, and was expressed as n moles of superoxide production per 106 cells. After 3 hr of incubation, cell-free supernatants were collected and stored at -20℃ until assayed for eosinophil degranulation. Degranulation was monitored by determining eosinophil- derived neurotoxin (EDN) concentrations in culture supernatants. EDN were measured by specific radioimmunoassay (RIA), as described previously [10]. All assays were performed in duplicate. In some experiments, to investigate whether enzyme activity of CP can induce eosinophil degranulation, the 27 kDa CP was pre-treated for 1 hr with a cysteine protease inhibitor (E-64) at room temperature before exposure to eosinophils. The concentration (10 µM) of E-64 used did not affect the viability of eosinophils. Data were expressed as mean ± SEM from 3 independent experiments with different donors. Statistical significance of differences between treatment and control groups were assessed by Student's t-test. A probability value of less than 0.05 was considered significant.
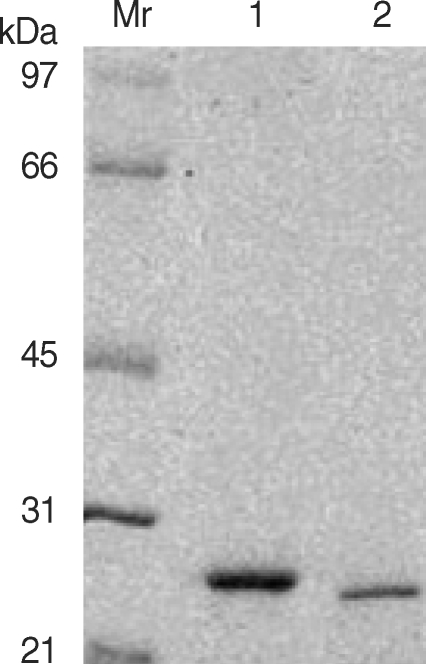
Electophoretic analysis of the purified 28 kDa (lane 1) and 27 kDa (lane 2) cysteine protease from the ESP using DEAE-trisacryl M anion-exchange column chromatography. Mr denotes relative molecular mass in kDa.
We checked whether each of the purified CP could activate eosinophils to induce superoxide production and degranulation. As shown in Fig. 2, incubation of eosinophils with purified 27 kDa CP induced superoxide production in time- and concentration-dependent fashions. The amount of superoxide production (16.3 ± 1.45 nmole) by eosinophils stimulated with 25 µg/ml of 27 kDa CP was comparable to that induced by 100 µg/ml of ESP from PwNEM as previously reported [10]. Furthermore, as shown in Fig. 3, we also found that 27 kDa CP highly induced eosinophil degranulation in a concentration-dependent manner. When eosinophils were incubated with 3.125, 6.25, 12.5, and 25 µg of 27 kDa CP/ml, EDN release (mean ± SEM) was 101.4 ± 13.4, 198.8 ± 25.9, 435.1 ± 82.0, and 758.4 ± 196.3 ng/106 cells, respectively. By contrast, as shown in Figs. 2 and 3, the purified 28 kDa CP showed minimal or no effects on superoxide production and degranulation even at the highest concentration (30 µg/ml) tested in this study. The amount of EDN release induced by the 28 kDa CP ranged from 38.9 ± 3.7 to 43.6 ± 4.4 ng/106 cells. The EDN release induced by medium was 28.4 ± 2.5 ng/106 cells. In order to determine the role of proteolytic activity of 27 kDa CP in eosinophil degranulation, the 27 kDa CP was pre-treated with a cysteine protease specific inhibitor E-64 before reaction with eosinophils. Although E-64 strongly inhibited the cleavage effect of 27 kDa CP on IgG, eosinophil degranulation induced by 27 kDa CP was slightly reduced by pre-treatment with E-64 (about 19% of controls) (data not shown), suggesting that enzyme activity of the 27 kDa CP is not the determining factor in the induction of eosinophil degranulation. Recently, we characterized in detail the functional role of protease activity of ESP by PwNEM [11]. For example, we showed that ESP secreted by PwNEM produced brain tissue injury by recruiting activated monocytes/microglia via heat-labile protease activity [11]. Interestingly, heat inactivation did not block the ability of ESP to induce microglial nitrous oxide (NO) production. Similarly, pre-treatment of ESP with thiol-crosslinking reagents strongly inhibited both proteolytic activity and cytotoxicity of ESP, but did not affect ESP-induced NO production in microglial cells [12]. These results suggest that the NO production and cytotoxicity triggered by ESP may be differentially regulated through unknown mechanisms, unrelated to cysteine protease activity. Therefore, further investigation is needed to clarify the exact mechanisms by which 27 kDa CP produced by PwNEM enhances eosinophil effecter functions.
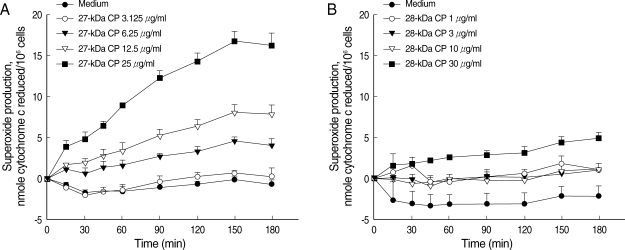
Superoxide anion production by eosinophils stimulated with purified 27 kDa or 28 kDa cysteine protease (CP) from the ESP produced by PwNEM. Eosinophils were stimulated with different concentrations of 27 kDa CP (3.125-25 µg/ml) (A), or 28 kDa CP (1-30 µg/ ml) (B), or medium alone for up to 3 hr at 37℃. Data are expressed as mean ± SEM from 3 independent experiments using different eosinophil donors.
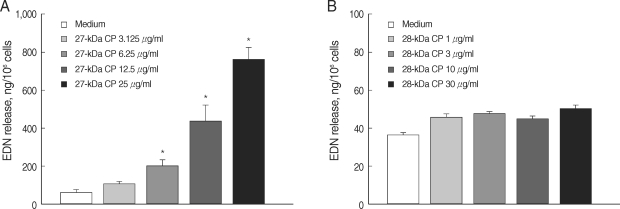
Degranulation of eosinophils stimulated with purified 27 or 28 kDa cysteine protease (CP) from the ESP produced by PwNEM. Eosinophils were stimulated with different concentrations of the 27 kDa CP (3.125-25 µg/ml) (A), 28 kDa CP (1-30 µg/ml) (B), or medium alone for 3 hr at 37℃. Data are presented as mean ± SEM of 3 independent experiments using different eosinophil donors. *Significantly different (P < 0.05) compared with the medium alone.
Although it has been speculated for a long time that cysteine proteases in ESP secreted by helminthic parasites might alter eosinophil function, the capacity of the cysteine proteases to directly induce eosinophil effecter functions has not been explored in detail. In the present study, we have demonstrated that 27 kDa, but not 28 kDa, CP plays an important role in eosinophil effecter functions, such as superoxide anion production and degranulation. The purified 27 kDa CP induced superoxide production from eosinophils in a time- and concentration-dependent manner. Moreover, it strongly induced eosinophil degranulation in a concentration-dependent fashion. In contrast, the 28 kDa CP purified from the ESP had no effect on eosinophil effecter function. What is the cause of the differences in effecter function of eosinophils induced by these 2 purified cysteine proteases from the ESP of PwNEM? Although we cannot answer to this question in the present study, it might be due to marked differences in enzymatic characteristics between the 2 kinds of proteases. On the basis of the substrate specificity, the 27 kDa CP purified from the ESP shared similarity with sparganum cathepsin S [13] or Fasciola hepatica cathepsin L [14], whereas the 28 kDa CP had mammalian cathepsin B-like properties [15]. A recent report has shown that the proteolytic activity of the 27 kDa CP against a Z-FR-AMC substrate was higher than that of the 28 kDa CP, and that the 28 kDa, but not the 27 kDa, CP directly facilitated tissue penetration by the PwNEM [2]. These collective data strongly suggest that these 2 cysteine proteses secreted by PwNEM have a different role in the host immune functions during tissue invasion in vivo.
The induction of degranulation of mature eosinophils by the 27, but not the 28 kDa, CP, produced by PwNEN may provide important clues to understanding of the pathogenesis of eosinophil-mediated tissue inflammatory reactions at worm-invaded lesions in helminthic infections. The ESP produced by PwNEM was recently found to rapidly induce a direct time- and concentration-dependent increase in the rate of constitutive apoptosis in normal human eosinophils [16]. Moreover, eosinophil apoptosis by the ESP was shown to be mediated by activation of caspase-3 [17]. In addition, we observed that 10 µg/ ml of the 27 kDa CP rapidly induced phosphatidylserine externalization on the cell surface of human eosinophils (data not shown). These results suggest that degranulation induced by the 27 kDa CP may result from an eosinophil cell death, which leads to eosinophil-mediated inflammation in larval-invaded lesions. In addition, proteases released from inflammatory cells, such as mast cells and neutrophils, can act as inflammatory mediators, leading to a tissue inflammation. For example, injection of human mast cell chymase into experimental animals resulted in a marked accumulation of neutrophils and eosinophils [18]. Inflammatory cell-derived proteases induce cytokine production and enhancement of effecter functions of human eosinophils [19,20]. Therefore, it is likely that worm-secreted proteases and inflammatory cell-derived proteases may synergistically act on eosinophils to increase the release of inflammatory mediators around worms in vivo.
In summary, we report that a 27 kDa cysteine protease in the ESP produced by PwNEM plays a crucial role in regulating effecter functions of human eosinophils. However, the exact role of other cysteine proteases of P. westermani remains to be elucidated. In addition to the 27 and 28 kDa CP of the metacercarial stage, there are many different cysteine proteases of maturing worms. There are at least 15 cysteine proteases among proteins of P. westermani adult ESP, with pH values between 4.5 and 8.5 and molecular weights between 27 and 35 kDa [21]. This unusual production of different cysteine proteases during worm development may be attributed to cross talk between hosts and worms in vivo. Therefore, it would be interesting to compare the effects of each protease produced by Paragonimus metacercariae, juveniles, and adult worms on eosinophil functions. These studies would help understanding of the pathophysiological roles of worm-secreted proteases in relation to eosinophil-associated tissue inflammations in helminthic diseases.
ACKNOWLEDGEMENTS
This study was supported by a grant no. HMP-02-PJ1-PG3-21299-0001 of the 2002 Good Health R&D Project, Ministry of Health & Welfare, Korea.