The Route of Leishmania tropica Infection Determines Disease Outcome and Protection against Leishmania major in BALB/c Mice
Article information
Abstract
Leishmania tropica is one of the causative agents of leishmaniasis in humans. Routes of infection have been reported to be an important variable for some species of Leishmania parasites. The role of this variable is not clear for L. tropica infection. The aim of this study was to explore the effects of route of L. tropica infection on the disease outcome and immunologic parameters in BALB/c mice. Two routes were used; subcutaneous in the footpad and intradermal in the ear. Mice were challenged by Leishmani major, after establishment of the L. tropica infection, to evaluate the level of protective immunity. Immune responses were assayed at week 1 and week 4 after challenge. The subcutaneous route in the footpad in comparison to the intradermal route in the ear induced significantly more protective immunity against L. major challenge, including higher delayed-type hypersensitivity responses, more rapid lesion resolution, lower parasite loads, and lower levels of IL-10. Our data showed that the route of infection in BALB/c model of L. tropica infection is an important variable and should be considered in developing an appropriate experimental model for L. tropica infections.
INTRODUCTION
Leishmania tropica is the causative agent of different forms of human leishmaniasis; anthroponotic, viscerotropic, and a few cases of visceral leishmaniasis [1]. There are few data available regarding factors that might be responsible for these vastly different clinical outcomes. Experimental models can be used to explore these factors. BALB/c mice have been proposed as an experimental model for these diseases [2,3]. Several variables have been reported to influence the immune responses as well as the disease outcome in experimental models of leishmaniasis. These variables include the developmental stage, dose, species, strain, and substrain of parasites used for injection, and alternative routes of inoculation [4,5].
More specifically, the route of infection has been reported to be an important variable in different experimental models for Leishmania parasites [6-8]. It was also reported that the route of infection with live Leishmania major vaccine has a critical role in the efficacy of vaccination [9]. The natural route of infection in leishmaniasis is the skin which contains unique cells such as dendritic cells and Langerhans cells with potent immunomodulatory effects. The skin environment is engaged in the natural route of infection. Subcutaneous injection in the footpad has been used in many reports for initiation of infection in experimental models of leishmaniasis. However, the subcutaneous route in the footpad may not be the natural route of infection in leishmaniasis because the level of engagement of skin environment in subcutaneous route may not be similar to the route of infection by the sandfly in nature. The intradermal injection in the ear has been proposed to be more similar to the natural route of infection in L. major experimental model [10]. It has been shown in a murine model of L. major that the subcutaneous route of infection in comparison to the intradermal route results in more potent immunity [9]. In a study on Leishmania donovani infection of BALB/c mice, it has been shown that a subcutaneous inoculation in comparison to an intradermal route resulted in more potent Th1 responses [11]. Data related to experimental models of L. tropica is limited. Whether the difference of the intradermal ear route and subcutaneous footpad route reported for L. major and L. donovani is valid for L. tropica is not clear. The effects of route of infection on disease outcome has not yet been reported for L. tropica.
In an attempt to mimic more closely the natural route of infection for L. tropica, we used the route of intradermal injection in the ear for initiation of the infection and compared it with the traditional route of subcutaneous injection in the footpad. The aim of this study was to explore the effects of route of infection on disease outcome and induction of protective immunity in the BALB/c model of L. tropica infection.
MATERIALS AND METHODS
Mice
BALB/c mice (females, 4-6 weeks old) were received from animal production facility of Pasteur Institute of Iran.
Parasites
L. tropica strain MHOM/AF/88/KK27 and L. major strain MRHO/IR/75/ER were used in this study. The internal transcribed spacer (ITS1) sequences of these Leishmania parasites confirmed their species as L. tropica (GenBank accession no. GQ913688.1) and L. major (GenBank accession no. GQ4719 00.1), respectively [12]. Stationary phase promastigotes were prepared as described elsewhere [2] and were used for all infections.
Injection methods
Intradermal injections were performed into the ear dermis. Subcutaneous injections were carried out into the footpad. Subcutaneous injections were done with great care to avoid the intramuscular or intradermal tissue. The injection volumes into the ears and footpads were 10 µl for immunizing infections. The injection volumes used for challenging infections were 50 µl into the footpad. Needles of 30 gauge were used for all injections.
Evaluation of disease outcome
Lesion development in the footpad and ear were used for assessment of disease outcome. The thicknesses of footpad or ear were measured by a dial-gauge caliper (Mitutoyu, Kawasaki, Kanagawa, Japan) at approximately bi-weekly intervals.
Assessment of delayed type hypersensitivity (DTH)
DTH was performed as reported elsewhere [13]. Briefly, DTH reaction was determined by measuring the footpad thickness at 18-24, 48, 72, and 96 hr after injection with L. major (challenging infection).
Parasite load assay
The loads of parasites were determined in the lymph nodes and spleen by culturing the homogenates of lymph node or spleen tissues in NNN media [14]. Parasite loads of the lymph node and spleen were determined in 3 mice per experimental group at 6 months after L. major challenge. Ten-fold serial dilutions were performed for extinction of the parasite growth. Assays were performed in duplicate. The parasite loads per total lymph node and spleen were calculated from the highest dilution at which promastigotes can be grown out.
Intracellular cytokine assay
Preparation and stimulation of lymph node cells, multicolor staining of cell surface markers and intracellular cytokines were performed as reported [13]. Briefly, single cell suspensions were made from the lymph node. The cells were stimulated with phorbol myristate acetate and ionomycine. Brefeldin A was used to inhibit protein trafficking through the Golgi. Fc receptors were blocked by anti-mouse CD16/CD32 (FcgII/III receptor). Saponin was used for permeabilization of the cell membrane. Intracellular cytokine staining was carried out by using anti-IL-10-PE. Cells were analyzed by Facscan flow cytometer (Beckton Dickinson, San Diego, California, USA) as well as Partec flow cytometer (Partec, Munster, Germany).
Statistical analysis
Comparisons of the footpad thickness, cell surface markers, and cytokine positive cells between experimental groups were performed by 1-tailed equal variance Student's t-test. P values equal or less than 0.05 were considered significant. Lymphocyte percentage was considered as a sample in statistical analyses.
Study design
Mice were infected by105 stationary promastigotes of L. tropica (as immunizing infection) by 2 methods; subcutaneous into the footpad and intradermal into the ear dermis. After the establishment of L. tropica infection, which took about 4-6 months, L. tropica pre-infected mice as well as naïve mice were challenged with 106 stationary promastigotes of L. major into the footpad subcutaneously. The contralateral footpad was used for challenging infection in mice which have already received the immunizing infection in 1 footpad. Evaluation of the immune response was carried out at 2 intervals; at week 1 and week 4 after L. major challenge. Lesion development was monitored throughout the experiment. Parasite loads of lymph nodes and spleens were determined 6 months after L. major challenge. The experiment was repeated once, and the results of the 2 experiments or 1 representative experiment are presented. The study was approved by the Ethical Committee of Pasteur Institute of Iran.
RESULTS
Outcome of primary L. tropica infection
Naïve BALB/c mice were infected by L. tropica through subcutaneous (into the footpad) or intradermal (into the ear dermis) routes. The subcutaneous injection of L. tropica resulted in a self-healing infection (Fig. 1). In contrast, intradermal injection resulted in a progressive and non-healing infection (Fig. 1).
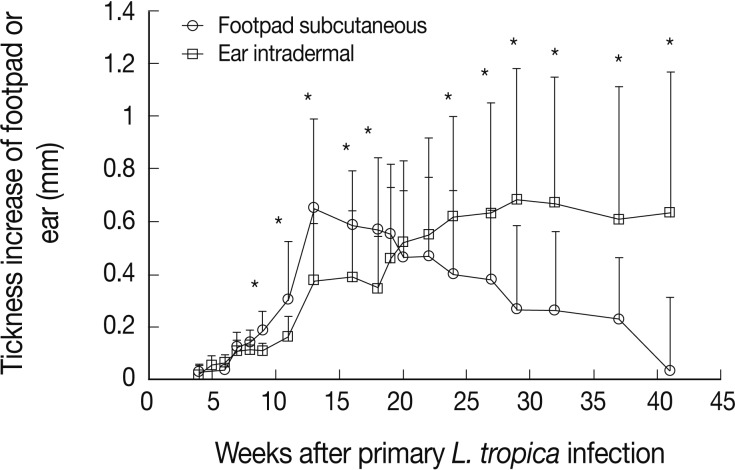
The disease outcome is influenced by the route of Leishmania tropica infection. BALB/c mice were infected by subcutaneous (into the footpad) or intradermal (into the ear dermis) inoculation of 105 stationary phase L. tropica promastigotes. Data represent the mean and SD of thickness increase of the footpad or ear of 6-15 mice per group from a representative experiment out of 2 replicate ones. Asterisks (*) show statistically significant difference (P<0.05) as calculated by the Student's t-test.
Outcome of L. major challenge
L. tropica pre-infected mice were challenged with L. major to evaluate the immune responses induced by L. tropica. L. major is a highly pathogenic parasite for BALB/c mice, which results in a progressive disease in naïve mice. Thus, the level of protective immunity could be assayed in vivo with confidence. The results showed that mice immunized through the footpad subcutaneous route, in comparison to the ear intradermal route, induced more protective immunity against L. major challenge resulting in lower footpad thicknesses from week 10 after challenge up to the end of the experiment (Fig. 2).
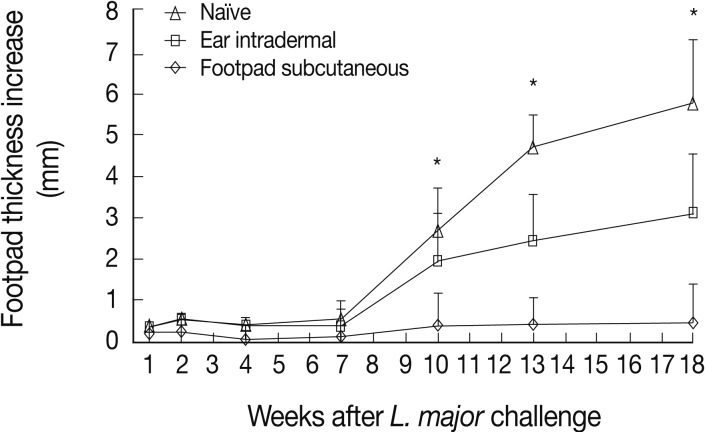
Lesion development after Leishmania major challenge in the footpad of L. tropica pre-infected BALB/c mice. Before challenge, mice were immunized by subcutaneous (into the footpad) or intradermal (into the ear dermis) inoculation of 105 stationary phase L. tropica promastigotes. Data represent the mean and SD of the footpad thickness for 6-15 mice per group from a representative experiment out of 2 replicate ones. Asterisks (*) show statistically significant difference (P<0.05) between subcutaneous vs. intradermal groups as calculated by the Student's t-test.
DTH
The DTH responses after L. major challenge are shown in Fig. 3. The highest DTH responses are seen in mice immunized by the footpad subcutaneous route, followed by the ear intradermal route. Negligible DTH responses were seen in naïve mice. The differences between the footpad subcutaneous route and ear intradermal route were statistically significant at all time points studied; 24, 48, and 72 hr post-challenge (P values were <0.002, <0.0004, and <0.01, respectively).
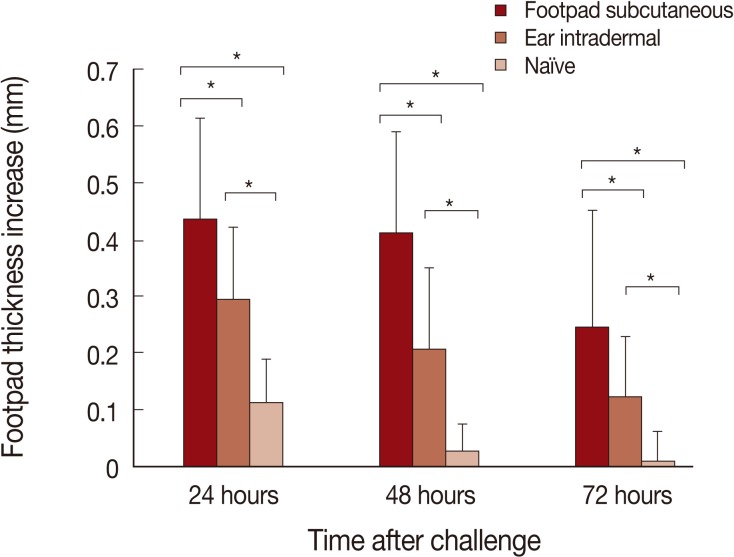
Delayed type hypersensitivity (DTH) responses to Leishmania major challenge are significantly higher in mice pre-infected by L. tropica through the footpad subcutaneous route in comparison to the ear intradermal route. Mice were pre-infected by L. tropica through subcutaneously in the footpad or intradermally in the ear. Mice were then challenged with L. major in the footpad. Data represent the mean and SD of the footpad thickness for 6-15 mice from a representative experiment out of 2 replicate ones. Asterisks (*) show statistically significant difference (P<0.05) as calculated by the Student's t-test.
Cytokine production
The route of injection with L. tropica has a critical role in IL-10 production. As shown in Fig. 4, the ear intradermal route in comparison to the footpad subcutaneous route resulted in a higher percentage of IL-10 producing lymphocytes in the lymph node at week 1 after challenge. No differences were detected between the 2 experimental groups of mice with regard to the percentages of IL-10 positive lymphocytes at week 4 after challenge (data not shown).
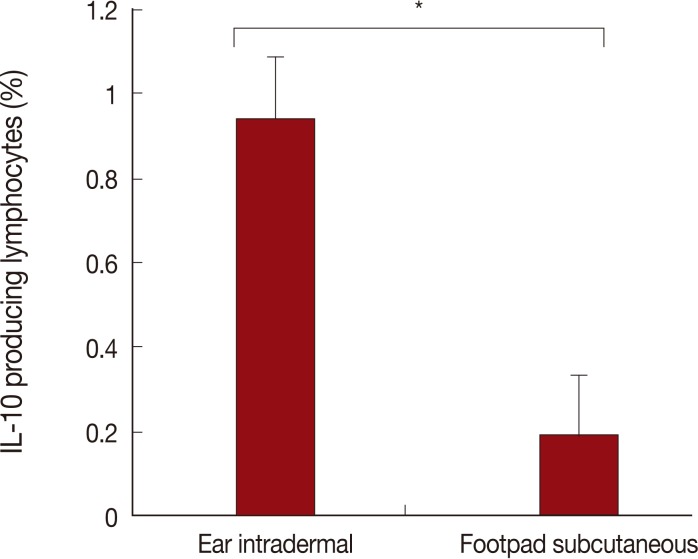
The percentage of IL-10 positive lymphocytes at week 1 after Leishmania major challenge in L. tropica pre-infected mice by ear intradermal or footpad subcutaneous routes. Data are mean and SD of percentages of IL-10 positive lymphocytes of 3 mice per group from 2 repeated experiments. The asterisk (*) shows statistically significant difference between the 2 groups.
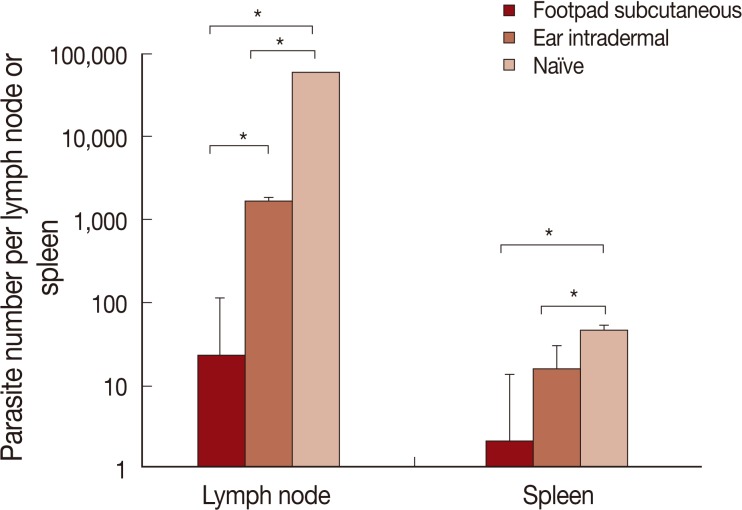
Parasite load
The results of parasite loads are shown in Fig. 5. Mice pre-infected with L. tropica through the footpad subcutaneous route, in comparison to the ear intradermal route, were more resistant to L. major challenge. The difference in parasite loads was statistically significant both in the lymph node and spleen.
DISCUSSION
While it is agreed that the route of immunization of protein antigens may also influence the development of an immune response, the effects of various routes on the level and type of immune responses generated and their subsequent impact on challenge infections have remained largely unknown [8]. The effect of route of injection on the immune response has been reported for immunization by particulate antigens [15,16]. A similar effect of route of infection has been reported for L. major [9] and L. donovani [11]. The effect of route of infection for L. tropica is considered in our study. We compared 2 infection routes for L. tropica; subcutaneous into the footpad and intradermal into the ear. The ear dermis was selected because it seems to be more close to the natural route of infection [10]. It is noteworthy that injection into the ear dermis is mainly intradermal due to the lack of any loose tissue under the skin dermis in the ear, and hence there is no possibility for subcutaneous injection in this site. The subcutaneous injection into the footpad has been frequently reported in the literature for initiation of cutaneous lesions in mice [17]. The advantages of this site include that lesion development can be evaluated by simple measurement of the footpad swelling, and that sequential measurements can be made on the same lesion, because they can be obtained without having to sacrifice the animal.
Our findings present a solid proof that the infection route of L. tropica has a critical role on the immune response of BALB/c mice against Leishmania infection. These effects determine the outcome of primary L. tropica infection as well as the level of protection against secondary L. major challenge. The intradermal ear route of infection of this parasite results in non-healing outcome while subcutaneous footpad route of infection of the same parasite resulted in a relatively self-healing outcome (Fig. 1). In addition, the intradermal ear route of L. tropica infection, in comparison to the subcutaneous footpad route, induced significantly less protective immune responses against secondary L. major challenge (Fig. 2), lower DTH response (Fig. 3) and higher levels of IL-10 responses at week 1 after L. major challenge (Fig. 4). These data showed that the intradermal ear route induces more suppressive immune responses in comparison to the subcutaneous footpad route resulting in higher parasite loads (Fig. 5). Our findings are the first for L. tropica and novel, hence extend our knowledge about the experimental model of L. tropica. In addition, our results have potential applications in vaccination strategies against L. tropica infections, as reported for L. major [9].
Why different routes of injections (subcutaneous vs. intradermal) result in different disease outcomes? It seems that the difference originates from the structure of different layers of the skin. The skin is composed of 3 primary layers from outside to inside; epidermis, dermis, and hypodermis. In contrast to the epidermis and dermis which contain many cells, blood, and lymphatic vessels, the hypodermis is naturally devoid of resident immune cells [18]. So, while the intradermal route targets dermal dendritic cells and macrophages, the subcutaneous injection do not directly involve the skin-resident antigen-presenting cells (Langerhans and dendritic cells), but depends instead on the presence of leukocyte infiltrates attracted by inflammatory molecules secreted at the vaccination site [18].
Our results proved the effects of the intradermal route of infection in suppressing the protective immunity against L. tropica in BALB/c mice. A similar suppressive role of epidermal cells was reported for L. major infection of C57BL/6 mice and the effect was ascribed to the expansion of T-reg cells induced by epidermal Langerhans cells [19]. Evidence from research on contact hypersensitivity has revealed that activation of Langerhans cells in the skin leads to induction of regulatory, immunosuppressive T-cells [20]. Our findings and other reports showed the relative suppressive role of the intradermal route in comparison to the subcutaneous route of injection. These findings suggest that the preferential injection route of live Leishmania vaccines, which may be basically similar to the wild type parasite, may be subcutaneous but not intradermal.
The effects of route of infection on immune responses have already been reported for L. major infection in C57BL/6 mice [9]. They showed that the route of L. major infection, the subcutaneous in the footpad vs. intradermal in the ear, could significantly influence the efficiency of the immune response. The subcutaneous infection in comparison to the intradermal resulted in higher antigen-specific IFN-γ producing T-cells, lower IL-10 producing T-cells, and a more rapid parasite clearance from the secondary challenge site [9]. It was also reported that more potent Th1 response is induced by the subcutaneous in comparison to the intradermal route of infection of L. donovani in BALB/c mice [11]. Our data are in agreement in most parts with these 2 reports. So, it seems that, in experimental models of leishmaniasis, the more suppressive immune responses induced by the intradermal ear route in comparison to the subcutaneous footpad route is a common feature for at least L. tropica and L. donovani infections of BALB/c as well as for L. major infection of C57BL/6 mice.
The site of infection has also been shown to influence the disease outcome in BALB/c mice [21,22]. The differences between the 2 infection routes of our study are 2 folds; the route of injection (subcutaneous vs. intradermal) as well as the site of infection (footpad vs. ear). Our data showed the combined effects of these 2 variables but were not conclusive about the separate effects of each of these 2 variables. Deciphering the separate effects of these 2 factors needs further study.
Our data confirmed that the route of infection in BALB/c mouse model of L. tropica infection is an important variable and should be considered in developing an appropriate experimental model for L. tropica infection. Many variables are responsible for protective immunity against L. tropica and route of infection is one of them. Our knowledge about these variables is very limited and more researches are needed to clarify its complexity in order to translate the experimental findings into a clinical use.
ACKNOWLEDGMENTS
We thank Dr. Mohammad Hossein Alimohammadian for his managerial assistance. The technical expertise and efforts of Davood Iravani, Mohammad Doroudian, and Maryam Moradi in experimental procedures are highly appreciated. This research received financial support from Pasteur Institute of Iran (Project No. 275).
Notes
Some part of this paper has been presented in "The 14th International Congress of Immunology", Japan, 2010.