Amoebic PI3K and PKC Is Required for Jurkat T Cell Death Induced by Entamoeba histolytica
Article information
Abstract
The enteric protozoan parasite Entamoeba histolytica is the causative agent of human amebiasis. During infection, adherence of E. histolytica through Gal/GalNAc lectin on the surface of the amoeba can induce caspase-3-dependent or -independent host cell death. Phosphorylinositol 3-kinase (PI3K) and protein kinase C (PKC) in E. histolytica play an important function in the adhesion, killing, or phagocytosis of target cells. In this study, we examined the role of amoebic PI3K and PKC in amoeba-induced apoptotic cell death in Jurkat T cells. When Jurkat T cells were incubated with E. histolytica trophozoites, phosphatidylserine (PS) externalization and DNA fragmentation in Jurkat cells were markedly increased compared to those of cells incubated with medium alone. However, when amoebae were pretreated with a PI3K inhibitor, wortmannin before being incubated with E. histolytica, E. histolytica-induced PS externalization and DNA fragmentation in Jurkat cells were significantly reduced compared to results for amoebae pretreated with DMSO. In addition, pretreatment of amoebae with a PKC inhibitor, staurosporine strongly inhibited Jurkat T cell death. However, E. histolytica-induced cleavage of caspase-3, -6, and -7 were not inhibited by pretreatment of amoebae with wortmannin or staurosporin. In addition, we found that amoebic PI3K and PKC have an important role on amoeba adhesion to host compartment. These results suggest that amebic PI3K and PKC activation may play an important role in caspase-independent cell death in Entamoeba-induced apoptosis.
INTRODUCTION
Entamoeba histolytica is an enteric protozoan tissue-invasive parasite causing amoebic colitis and occasionally liver abscess in human beings. In developing country, this invasive parasite can infect 50 million and kill 100,000 people annually [1]. E. histolytica has a capability to induce apoptotic cell death in various host immune cells such as T lymphocytes and neutrophils [2], which results in not only minimizing host tissue damage but also maintaining parasite's infectivity and virulence in vivo. E. histolytica-induced host cell apoptosis results from amoebic adherence to host cells via Gal/GalNAc lectin on the surface of the amoeba [3]. The apoptosis induced by E. histolytica was almost completely inhibited by the addition of D (+)-galactose in Jurkat T cells [4]. In addition, mammalian cells lacking N-terminal Gal and GalNAc protein modifications are resistant to amebic killing [5,6], suggesting that amoebic contact to host cells is very critical process. E. histolytica can kill Jurkat leukemia T cells in vitro by induction of caspase 3-dependent apoptosis [3,7] or caspase-independent death mechanisms including protein tyrosine dephosphorylation and ROS generation in Jurkat T cells [8,9].
Phosphorylinositol 3-kinase (PI3K) catalyzes the phoshorylation of inositol phospholipids as well as at position 3, to generate phosphatidylinositol 3,4,5-triposphate [PI(3,4,5)P3], via PI 3-monophosphate and PI 3,4-bisphosphate. These lipid products bind specific protein molecules for the manifestation of various cellular functions, including cell adhesion, vesicular trafficking, endocytosis, chemotaxis, degranulation, actin rearrangement, cell differentiation, cell growth, and cell survival [10,11]. Selective PI3K inhibitors such as wortmannin, and LY294002 have been developed that reduce inflammation and some characteristics of disease in experimental animal model [12,13]. In E. histolytica, at least 3 potential PI3K orthologues have been found in searching for matches in the TIGR institute database [14]. Specially, wortmannin, a potent inhibitor of PI3K family of enzymes, markedly inhibited phagocytosis by E. histolytica of bacteria, red blood cells and mucin-coated beads [15], suggesting an important role of PI3K in phagocytosis by this parasite. In addition, wortmannin decreases the proteolytic activity of E. histolytica and ALA development [16]. However, the role of amoebic PI3K in amoeba-induced apoptosis and cell death signal pathway is poorly understood.
Protein kinase C (PKC), a phosholipid-dependent serine/threonine kinase, plays crucial roles in signal transduction for various cellular responses, including cell proliferation, survival, and apoptosis [17]. Like many PKC isoforms existed in mammalian cells, PKC activity was demonstrated in various stains of E. histolytica [18]. However, in E. histolytica, there is little information on the presence of PKC isozymes and their function. A partially purified PKC preparation from E. histolytica was shown to phosphorylate histone in the presence of calcium, phospholipid, and DAG [18]. Use of PKC inhibitors demonstrates that PKC plays an important role in actin cytoskeleton for motility and adhesion to target cells by E. histolytica [19,20]. In addition, this PKC activity is involved in the process of erythrophagocytotis by E. histolytica [30] and relocalized to the membrane during phagocytosis [18]. Moreover, activation of PKC appears to induce vesicle exocytosis following Entamoeba-fibronectin interaction [20]. However, the role of amoebic PKC in amoeba-induced apoptosis and cell death signal pathway is poorly understood.
In this study, we investigated whether Jurkat T cell death signaling events induced by E. histolytica are associated with amoebic PI3K and PKC using various PI3K inhibitors or PKC inhibitors.
MATERIALS AND METHODS
Reagents
PI3K potent inhibitor wortmannin, LY294002 and PKC inhibitor staurosporin, Rottlerin and Ro-31-8220 were all purchased from Calbiochem (La Jolla, California, USA). ECM components such as laminin (from human placenta) and collagen I (from human placenta), and crystal violet were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Fluorescein isothiocyanate (FITC)-labeled annexin V was purchased from BD Pharmingen (San Diego, California, USA). Rabbit polyclonal Abs against caspase-3, -6, -7, and β-actin were purchased from Cell signaling technology (Beverly, Massachusetts, USA). HRP-conjugated anti-rabbit IgG polyclonal Ab against caspases and β-actin were purchased from Cell signaling technology (Beverly).
Cultivation of E. histolytica and Jurkat T cells
Trophozoites of E. histolytica (HM1:IMSS) were grown axenically in TYI-S-33 medium at 37℃. Trophozoites were harvested after 48 to 72 hr during the logarithmic phase by chilling the culture tubes on ice for 10 min. After centrifugation at 200×g at 4℃ for 5 min, the trophozoites were washed with RPMI 1640 medium supplemented with 25 mM HEPES, 50 mg/L gentamicin sulfate, and 10% (v/v) heat-inactivated fetal bovine serum (FBS), and were suspended in the culture medium.
The human leukemia T-cell line Jurkat-E6-1 (American Type Culture Collection) was grown in RPMI 1640 medium (Invitrogen, Carlsbad, California, USA) supplemented with 25 mM HEPES, 50 mg/L gentamicin sulfate, and 10% (v/v) heat-inactivated fetal bovine serum (FBS) at 37℃ in a humidified 5% CO2 atmosphere. The viability of the amebae and Jurkat, as judged by trypan blue exclusion test, was always shown to be 99%.
Pretreatment of E. histolytica with PI3K or PKC inhibitors
E. histolytica were pretreated with various concentration of wortmannin (1-500 nM), LY294002 (1-200 µM), staurosporine (10-100 nM), Rottlerin (5-50 µM) Ro-31-8220 (1 or 10 µM) or 1% DMSO as a control for 20 min at 37℃. After pre-incubation with the inhibitors for the indicated time, E. histolytica were washed twice with culture medium before they were co-incubated with Jurkat T cells. No cytotoxicity of all inhibitors at the concentration tested was observed.
E. histolytica adhesion assay
In order to assess E. histolytica cell adhesion to ECM protein, 96-well flat-bottom plates were coated with laminin (5 µg/ml), collagen I (5 µg/ml), fibronectin (5 µg/ml) or BSA (5 µg/ml) at 37℃ for 2 hr. Nonspecific binding sites were blocked by subsequent incubation with 1%BSA in Hank's balanced solution (HBSS) for 1 hr. E. histolytica (2×104/well) pretreated with or without various inhibitors were then seeded into the coated 96-well flat-bottom plates and incubated for 1 or 5 min at 37℃. After incubation, unbound amoebae were washed off with PBS and those adhered were fixed with 1% formalin for 10 min. Amoebae that adhered to the well plate were stained with crystal violet. To remove remaining dye, plates washed off with PBS twice. After then, dye bound to adhered amoebae was solubilized with 1% SDS, and absorbance was measured at 570 nm using VERSAmax microplate reader (Molecular Devices Inc., Sunnyvale, California, USA).
Measurement of PS externalization in Jurkat T cells by flow cytometry
The apoptosis in Jurkat T cells was quantitated by examining the percentage of cells with annexin V bindings on the cell surfaces. Jurkat cells (4×105/well) were seeded into 48-well tissue culture plate and the preteated-trophozoites with or without various inhibitors were added each well. Jurkat cells and trophozoites were incubated a ratio of 10:1 for 30 or 60 min at 37℃ in a humidified CO2 incubator (5% CO2 and 95% air atmosphere). After incubation, cells were washing once with PBS and stained with FITC-conjugated annexin V for flow cytometric measurement. Data are presented as mean±SEM from 3 independent experiments. Significant differences from the value obtained with cells incubated with medium alone are shown (P<0.05).
Analysis of DNA fragmentation in Jurkat T cells by agarose gel electrophoresis
To assay DNA fragmentation in Jurkat cells by amoebic-PI3K and PKC, Jurkat T cells (5×106/group) were incubated with trophozoites of pretreated E. histolytica (5×105/group) with or without inhibitors at 37℃ for 60 min in a humidified CO2 incubator. To elucidate the contact dependency of host cell killing by the ameba, Jurkat cells were incubated for 60 min with E. histolytica trophozoites. After incubation, the cells were harvested by centrifugation. The cells were washed with PBS. DNA was extracted using the lysis buffer (1% NP-40 in 20 mM EDTA, 50 mM Tris-HCl, pH7.5; 10 µl per 106 cells, minimum 50 µl). After centrifugation for 5 min at 1,600 g, the supernatant was collected and treated with RNAse A for 2 hr at 56℃ and brought to 1% (w/v) SDS, followed by digestion with proteinase K (final concentration 2.5 µg/µl) for at least 2 hr at 37℃. DNA was then precipitated and analysed by gel electrophoresis in 1% agarose gel, and visualized by staining with ethidium bromide.
Immunoblotting
Jurkat cells (1×106/group) were incubated with or without trophozoites of E. histolytica (1×105/group). Trophozoites pretreated with or without various pharmacologic inhibitors were incubated with Jurkat cells. After incubation for the indicated times, the reaction was stopped by brief centrifugation. The cells were lysed with lysis buffer (20 mM Tris-HCl pH7.5, 60 mM β-glycerophosphate, 10 mM EDTA, 10 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol, 1 mM Na2VO4, 1 mM APMSF, 1% NP-40, and 5 µg/ml leupeptin) on ice for 30 min. After centrifugation at 12,000 g for 5 min, the supernatants were saved, diluted in SDS-PAGE loading buffer, and heated at 100℃ for 5 min. The samples were stored at -20℃ until use. Samples were subjected to 12%, or 15% SDS polyacrylamide gels and were electrotransferred onto Immobilon P polyvinylidene fluoride membranes (EMD Millipore corporation, Billerica, Massachusetts, USA). The membranes were blocked with 5% nonfat dry milk in TBST at room temperature for 1 hr and then incubated with primary Abs against caspase-3, -6, and -7, or calpain regulatory subunit at 4℃ overnight. The membranes were subsequently soaked with HRP-conjugated anti-rabbit IgG at room temperature for 1 hr. Immunoreactivity was detected using LumiGLO (Cell Signaling Technology, Beverly).
Statistical analysis
Results were expressed as the mean±SEM from 3-5 independent experiments. One-way analysis of variance (ANOVA) was performed using the statistical software package SPSS version 20 for window. The post-hoc comparisons of means from different groups were made by the Bonferroni post-hoc test. Values were considered statistically significant when P-value≤0.05.
RESULTS
Pretreatment of E. histolytica with a PI3K inhibitor wortmannin inhibits Entamoeba-induced PS externalization and DNA fragmentation in Jurkat T cells
To investigate whether PS externalization of Jurkat T cells induced by E. histolytica is associated with amoebic PI3K, we examined the inhibitory effect of 2 PI3K inhibitors such as wortmannin and LY294002. As shown in Fig. 1A, when Jurkat cells were incubated with E. histolytica trophozoites for 30 and 60 min, the percentage of cells stained with annexin V was 14.15±2.0 and 31.11±6.3, respectively. However, pretreatment of E. histolytica with wortmannin (50 or 100 nM) for 30 min was inhibited in induction of PS externalization in Jurkat T cells in a concentration-dependent manner compared to results for DMSO-pretreated amoebae. In contrast, pretreatment of another PI3K inhibitor LY294002 had no significant difference compared with the control (Fig. 1B). Next, we investigated the role of amoebic PI3K on Entamoeba-induced DNA fragmentation of Jurkat T cells. As shown in Fig. 2A, when Jurkat cells were incubated for 1 hr with wortmannin-pretreated Entamoeba, DNA fragmentation in Jurkat T cells was inhibited at a 100 and 200 nM concentration of wortmannin. However, LY294002 did not inhibit DNA fragmentation at any concentration (Fig. 2B).

Pretreatment of E. histolytica with PI3K inhibitor (wortmannin) but not LY294002 reduces externalization of phosphatidylserine (PS) in Jurkat T cells. E. histolytica (4×104/well) were pretreated with PI3K inhibitors or 1% DMSO as a control for 20 min at 37℃. Then, Jurkat T cells (4×105/well) were incubated for 30 or 60 min at 37℃ with or without E. histolytica (4×104/well). After incubation, cells were stained with FITC-conjugated annexin V for flow cytometric measurement. All reactions were performed in triplicate. All data are presented as the mean±SEM of at least 3 independent experiments. Significant differences from the value obtained with cells incubated with medium alone are shown. *P<0.05.
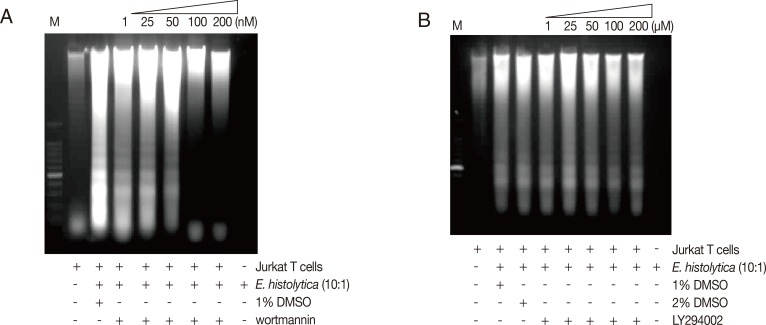
Treatment of E. histolytica with PI3K inhibitor, wortmannin results in the reduction of Entamoeba-induced DNA fragmentation. Pretreated E. histolytica (4×105/sample) with or without wortmannin, LY294002 for 20 min at 37℃ were incubated for 60 min at 37℃ with Jurkat T cells (4×106/sample) in the absence or presence of E. histolytica (4×105/sample) in a CO2 incubator. DNA fragmentation was analyzed by 2% agarose gel electrophoresis. An equal number of amebae and Jurkat T cells were incubated in medium alone as negative controls.
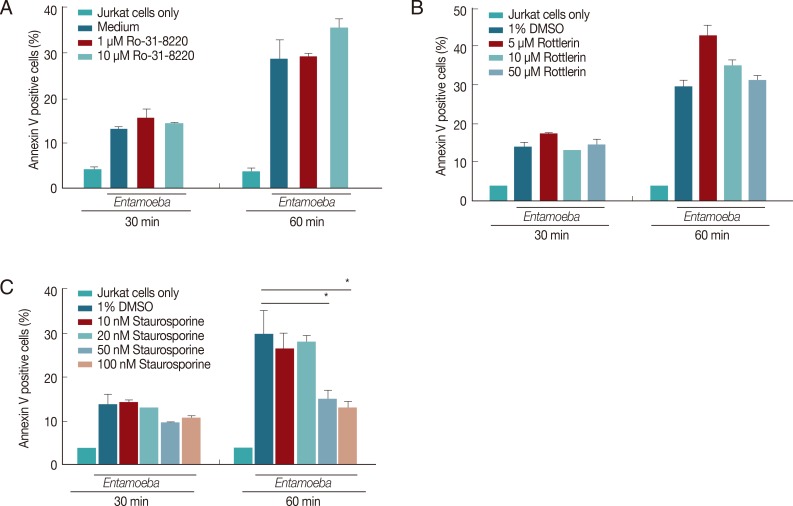
Pretreatment of E. histolytica with a PKC Inhibitor staurosporine inhibits on Entamoeba-induced PS externalization and DNA fragmentation in Jurkat T cells
To know if amebic PKC plays a role in Entamoeba-induced PS externalization and DNA fragmentation in Jurkat T cell, we used various PKC inhibitors such as Ro-31-8220, rottlerin, and staurosporine. As shown in Fig. 3A, E. histolytica-induced PS externalization was effectively inhibited by pretreatment of amoebae with staurosporine compared to the result of DMSO-pretreated amoebae. For example, at 60 min after incubation, the percentage of annexin V-positive cells co-incubated with staurosporine-pretreated amoebae was 17.2±1.2 (50 nM) and 14.4±2.0 (100 nM), respectively. At that time, the percentage of annexin V-positive cells incubated with DMSO-treated amoebae showed 29.5±3.5. In contrast, pretreatment of amoebae with other PKC inhibitors such as rottlerin or Ro-31-8220 did not effectively reduce PS externalization (Fig. 3B, C). Next, we examined the effect of amoebic PKC on DNA fragmentation in Jurkat T cells induced by E. histolytica, E. histolytica were pretreated with various PKC inhibitors such as staurosporine, Ro-31-8220 and rottlerin for 20 min before being incubated with Jurkat cells. As shown in Fig. 4A, when Jurkat cells were incubated for 1 hr with staurosporine pretreated Entamoeba, DNA laddering is partially inhibited at a concentration of 200 nM compared to the result for medium control. However, other PKC inhibitors; rottlerin and Ro-31-8220 have no inhibitory effect on Jurkat T cells DNA fragmentation at any concentration (Fig. 4B, C).
PKC inhibitors staurosporine result in the reduction of E. histolytica-induced PS externalization. E. histolytica (4×104/well) were pretreated with staurosporin, rottlerin, Ro-31-8220, or 1% DMSO as a control for 20 min at 37℃. Then, Jurkat T cells (4×105/well) were incubated for 30 or 60 min at 37℃ with or without E. histolytica (4×104/well). After incubation, cells were stained with FITC-conjugated annexin V for flow cytometric measurement. All reactions were performed in triplicates. Data are presented as mean±SEM from 3 independent experiments. Significant differences from the value obtained with cells incubated with medium alone are shown. *P<0.05.
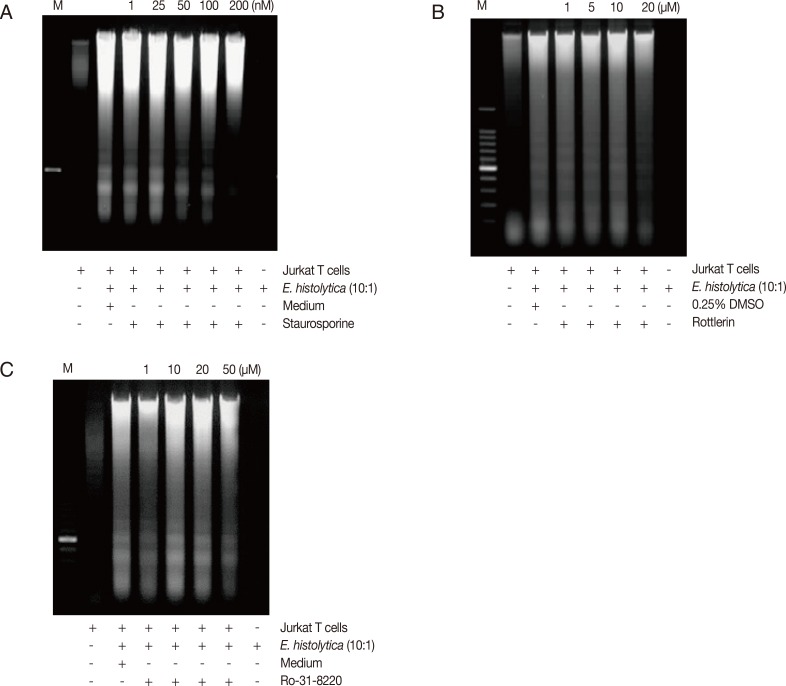
E. histolytica pretreated with staurosporine inhibits DNA fragmentation in Jurkat T cells. Pretreated E. histolytica (4×105/sample) with or without Staurosporine, Rottlerin, and Ro-31-8220 for 20 min at 37℃ were incubated for 60 min at 37℃ with Jurkat T cells (4×106/sample) in the absence or presence of E. histolytica (4×105/sample) in a CO2 incubator. DNA fragmentation was analyzed by 2% agarose gel electrophoresis. An equal number of amebae and Jurkat T cells were incubated in medium alone as negative controls.
Pretreatment of E. histolytica with PI3K or PKC inhibitors does not inhibit cleavage of caspase 3, 6, and 7 in Jurkat T cells induced by E. histolytica
To examine the role of amoebic PI3K and PKC in the activation of caspases in Jurkat T cell apoptosis induced by E. histolytica, we pretreated Entameoba with PI3K or PKC inhibitors. Proteolytically activated forms of caspase-3, -6 or -7 were detected at 15 min after incubation with E. histolytica. As shown in Fig. 5A and B, when Jurkat cells were co-incubated for 15 min with 100 nM wortmannin-pretreated Entamoeba, amebic-induced cleavage of caspases were partially inhibited compared to DMSO-treated amoebae. Another PI3K inhibitor LY294002 also did not show any inhibitory effect on Entameoba-induced cleavage of caspases (Fig. 5B). As shown in Fig. 6, cleavage of caspases in Jurkat T cells induced by amoebae pretreated with PKC inhibitors such as staurosporine, rottlerin, or Ro-31-8220 were not inhibited by at any concentration of inhibitors compared to the result of DMSO-pretreated amoebae.
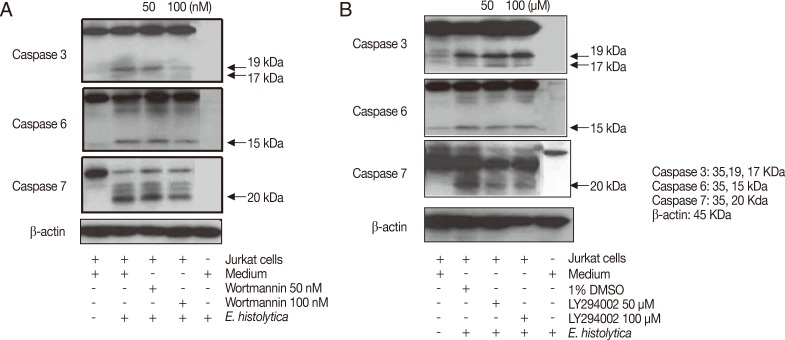
Pretreatment of Entamoeba histolytica with PI3K inhibitors induces cleavage of caspase 3, 6, and 7 in Jurkat T cells. Pretreated E. histolytica (1×105/sample) with or without various PI3K inhibitors for 20 min at 37℃ were incubated with Jurkat T cells (1×106/sample) at a 10:1 cell ratio (Jurkat T cells to E. histolytica) for 15 min at 37℃ in a CO2 incubator. After incubation, whole cell lysates were subjected to SDS-PAGE and blotted with anti-caspase 3, 6, 7, and β-actin Ab. The figure is representative of 3 experiments showing similar results.
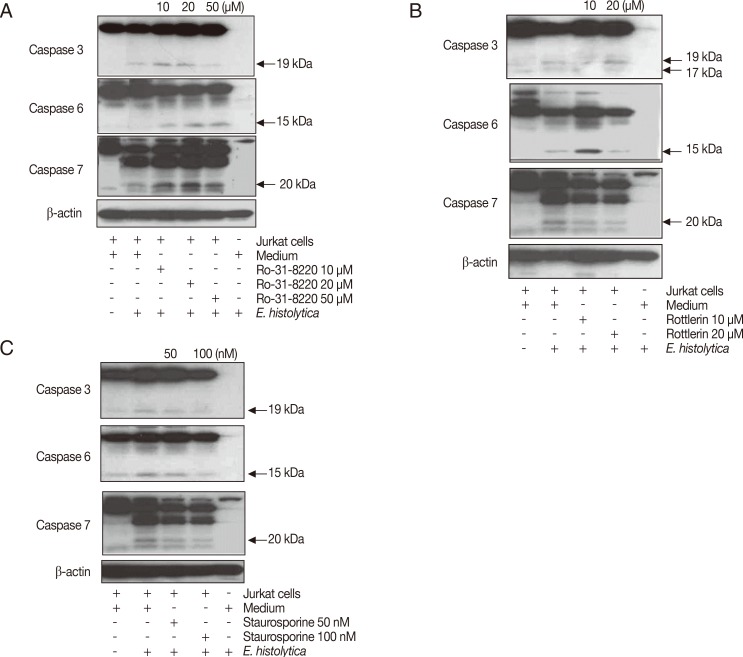
Pretreatment of Entamoeba histolytica with PKC inhibitors induces cleavage of caspase 3, 6, and 7 in Jurkat T cells. Pretreated E. histolytica (1×105/sample) with or without Staurosporine, Rottlerin, and Ro-31-8220 for 20 min at 37℃ were incubated with Jurkat T cells (1×106/sample) at a 10:1 cell ratio (Jurkat T cells to E. histolytica) for 15 min at 37℃ in a CO2 incubator. After incubation, whole cell lysates were subjected to SDS-PAGE and blotted with anti-caspase 3, 6, 7, and β-actin Ab. The figure is representative of 3 experiments showing similar results.
Pretreatment of E. histolytica with wortmannin or staurosporine inhibits amoebic adhesion to host compartment
To investigate whether amoebic PI3K and PKC can regulate amoeba adhesion to host compartment like laminin or collagen, amoebae pretreated with various PI3K or PKC inhibitors for 20 min were seeded into ECM-coated plates. As shown in Fig. 7, a significant decrease in amoebic adherence to laminin and collagen was observed when wild-type amoebae were pretreated with wortmannin or staurosporine. PI3K inhibitor, 250 nM wortmannin resulted in a 49.7% and 37.0% inhibition of amoeba adhesion to laminin and collagen, respectively. PKC inhibitor, 100 nM staurosporine showed the significant inhibition of amoeba adhesion to laminin (40% inhibition) and collagen (50% inhibition), respectively. However, pretreatment with other PI3K inhibitor LY294002 or PKC inhibitors did not shown any inhibitory effect on amoeba adhesion to ECM.
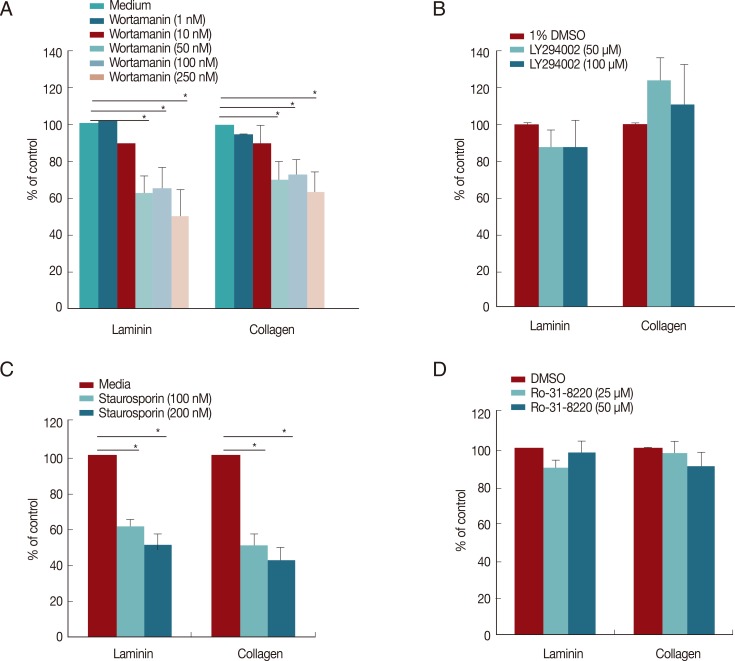
Pretreatment of E. histolytica with wortmannin or staurosporine reduces amoebic adhesion to ECM proteins. Pretreated amoebae with wortmannin (A), LY294002 (B), staurosporine (C), or Ro-31-8220 (D) for 20 min were incubated for 5 min after exposure to laminin or collagen. All reactions were performed in duplicates. Data are presented as the mean±SEM of 3 independent experiments. *P<0.05, indicating significant differences compared to controls.
DISCUSSION
In this study, we investigated the role of amebic PI3K and PKC on Jurkat T cell death using various pharmacological PI3K and PKC inhibitors. Pretreatment of E. histolytica with PI3K inhibitor, wortmannin, not LY294002 had an effect on preventing PS externalization and DNA fragmentation of Jurkat T cells by E. histolytica and amoebic adhesion. It is thought that this difference of inhibition between wortmannin and LY294002 was caused by different actions. Wortmannin is irreversibly inhibited by the cell-permeable fungal protein wortmannin, which covalently modifies Lys-802 in the catalytic subunit [22]. The morpholino derivative LY294002 is a reversible inhibitor that acts on the ATP-biding site of PI3K [23]. According to published papers, wortmannin-treated amoeba showed the decrease of virulence compared to untreated-amoeba. For examples; Pretreated E. histolytica with wortmannin completely inhibited amoeba movement [24], phagocytosis [15] and amoebic protease activity [16]. In addition, chemotaxis of E. histolytica towards TNF (but not motility) was inhibited by treatment with PI3K inhibitor, wortmannin, suggesting that PI3Ks were an important element in the regulation of chemotatic signaling [40]. Interestingly, Gal/GalNAc lectin genes, hgl and lgl, were also up-regulated during chemotaxis. Moreover, a chemotaxis migration assay demonstrated that trophozoites inhibited for Gal/GalNAc adhesion-activities were impeded for chemotaxis [40].
In present study, pretreated amoeba with wortmannin showed the reduced adhesion to ECM proteins compared to vehicle-treated amoeba or LY294002 treated amoeba. These results suggest that amoebic PI3K is closely related to amoebic adhesion to target cells. Amoebic PI3K might be involved in trafficking to and assembly in the plasma membrane of Gal/GalNAc lectin. Gal/GalNAc lectin usually resides in the lipid rafts of the plasma membrane and intracellular site [34]. Gal/GalNAc lectin is a heterodimer composed of a heavy chain (170 kDa, Hgl) and an extracellular light chain (31-35 kDa, Lgl) linked by disulfide bonds [2]. Adherence to target cells via Gal/GalNAc lectin is known to be required for host cell contact-dependent cytotoxicity caused by E. histolytica. This process of E. histolytica is central to pathogenesis and it is known to be driven by the motility of the parasites [21]. In this study, we observed wortmannin-treated Entamoeba has much more round shape than vehicle-treated Entamoeba (data not shown), indicating the decrease of motility. Therefore, wortmannin-treated Entamoeba appears not to have ability anymore to maintain a persistent directional cell polarization and cell migration.
The activation of PKC is an important step for trophozoites adhesion, actin polymerization and reorganization, protein phosphorylation, and increased local proteolytic activity [20]. Although signaling mechanisms have been unknown to parasitic amoebas, it is reported a stimulatory effect of phorbol esters on the pathogenic capacity of E. histolytica toxicity and suggested a role for Ca2+ and protein kinase C (PKC) in the adhesion and killing of target cells [19,25].
In present study, pretreatment of E. histolytica with PKC inhibitor, staurosporine had an effect on preventing PS externalization and DNA fragmentation of Jurkat T cells by E. histolytica and amoebic adhesion to ECM protein.PKC activity and PKC homologous gene fragments were found in E. histolytica [18,26]. PKC-dependent phosphorylation of proteins that regulates actin organization has been reported to occur at contact sites between the cells and the ECM substrates [27]. Staurosporine, which compete for the ATP binding site in PKC, reduced the adhesion to fibronectin in a concentration dependent manner [20]. Our results that staurosporine-treated amoeba inhibited Jurkat T cell death, agree with the previous data that PKC plays an important role in the adhesion and killing of target cells by E. histolytica [19,20]. However, other PKC inhibitors have no effect. The reason of this difference is not clear. These differences might be caused by action mechanism of inhibitors. Although there were other research used various PKC inhibitors in E. histolytica, only broad and potent PKC inhibitor, staurosporine induced the inhibition of amoebic PKC activity. Otherwise, amoebic PKC might be more primitive than mammalian PKC. These results may be caused by the difference of PKC structure between amoebic PKC and mammalian PKC. Although amoeba trophozoites require the integrity and functionality of the actin cytoskeleton for motility and adhesion, processes dependent on PKC activity [28], PKC of Entamoeba was not yet revealed their isoforms. In mammalian, the PKC family comprises at least 12 isoenzymes of serine/threonine protein kinases with a broad range of tissue distribution and differential cellular localization [29]. These PKC isozymes have been classified into 3 groups on the basis of their structure and the ability to bind cofactors [30]. PKC isozymes α, β, γ are known as conventional PKC (cPKC) and are activated in the presence of Ca2+, phosphatidylserine (PS), and diacylglycerol (DAG). PKC isozymes δ, ε, θ, and µ, are known as novel PKCs (nPKC) and these PKC isozymes are calcium independent but require DAG and PS for activation. Atypical PKC isozymes (ζ, λ, and ι) are not dependent on DAG for activation and are therefore not down-regulated by phorbol esters.
Activation of PKC through binding of fibronectin or direct stimulation with phorbol-myristate acetate also shows a direct relationship with actin polymerization and of various actin binding proteins in the adhesion from E. histolytica [31]. It has also been suggested that the Gal/GalNAc perhaps transmits signals within E. histolytica trophozoites to activate its protein kinase C which in turn stimulates amebic cytolytic activity [32]. Because the movement of these Gal/GalNAc lectin to plasma membrane is closely related to PKC and PI3K [33,35,36,37], wortmannin and staurosporine-treated amoebae had the defect on amoebic adhesion to target cells. Further research on the signaling mechanism of amoebic PI3K and PKC in the activation of Gal/GalNAc lectin in the adherence of amoebae to host factors is required.
In this study, we examined the effect of protein kinase C (PKC) and PI3K, which are signaling molecules responsible for numerous cellular responses, on the Jurkat T cell death induced by E. histolytica. Jurkat cell death and amoebic adhesion to ECM proteins were inhibited by pretreatment of amoebae with the PKC inhibitor staurosporine and PI3K inhibitor wortmannin in a concentration-dependent manner. These results indicate that signaling through PKC and PI3K in E. histolytica may contribute to the Jurkat T cell death induced by E. histolytica.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (Numbers 2012R1A2A2A02015054). Young Ah Lee was supported by post-doctorial fellowship of the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (355-2011-1-E00057).
Notes
We have no conflict of interest related to this study.