Serine Proteases of Parasitic Helminths
Article information
Abstract
Serine proteases form one of the most important families of enzymes and perform significant functions in a broad range of biological processes, such as intra- and extracellular protein metabolism, digestion, blood coagulation, regulation of development, and fertilization. A number of serine proteases have been identified in parasitic helminths that have putative roles in parasite development and nutrition, host tissues and cell invasion, anticoagulation, and immune evasion. In this review, we described the serine proteases that have been identified in parasitic helminths, including nematodes (Trichinella spiralis, T. pseudospiralis, Trichuris muris, Anisakis simplex, Ascaris suum, Onchocerca volvulus, O. lienalis, Brugia malayi, Ancylostoma caninum, and Steinernema carpocapsae), cestodes (Spirometra mansoni, Echinococcus granulosus, and Schistocephalus solidus), and trematodes (Fasciola hepatica, F. gigantica, and Schistosoma mansoni). Moreover, the possible biological functions of these serine proteases in the endogenous biological phenomena of these parasites and in the host-parasite interaction were also discussed.
INTRODUCTION
Proteases are a type of enzymes that are widely distributed in nature and are found ubiquitously in eukaryotes, prokaryotes, and viruses. They perform proteolytic reactions and peptide bond hydrolysis. Based on the important chemical groups in their active sites, proteases are typically categorized as 4 major classes (e.g., serine, metallo-, cysteine, and aspartic proteases). Of these classes, serine proteases are receiving increasing attention due to their diverse array of functions. They are involved in various aspects of physiological progression, such as digestion, apoptosis, signal transduction, blood coagulation, and wound healing through the proteolysis cascade action [1]. Aside from their roles in the physiology of organisms, they also play crucial roles in the pathogenesis of a number of diseases, such as cardiopulmonary disease and emphysema [2].
Parasitic helminths are one of the most important pathogens worldwide and are classified into nematodes (roundworms), trematodes (flatworms), and cestodes (tapeworms). Humans are constantly threatened by infections with these pathogens, which cause a wide variety of infectious diseases. During the process of infection, proteases derived from parasites are thought to be significant factors for successfully establishing infection. Experimental evidence has shown that serine proteases are involved in a wide variety of events in the life cycle of helminths.
The vast majority of serine proteases are digestive proteases involved in metabolic food processing or host tissue penetration. Additional serine proteases that are involved in reproduction, evasion of the host immune system, and development have also been characterized [3]. The essential roles of parasite serine proteases and their diverse activities make them attractive targets for the development of novel immunotherapeutic, chemotherapeutic, and serodiagnostic agents for the next generation of antiparasite interventions. The molecular and biochemical characterization of the serine proteases derived from these parasites is therefore central to the understanding of helminth-host interplay and the successful control of helminth infections. In this review, we summarized what is known of helminth serine proteases and their putative functions.
SERINE PROTEASES AND ENZYME MECHANISMS
Serine proteases are named because of the presence of a nucleophilic serine residue at the active site. The serine residue plays important roles in mediating protein hydrolysis. Most members of the serine proteases contain 3 essential residues at their active sites: a serine (Ser), a histidine (His), and an aspartate (Asp). Although these 3 residues do not have continual distribution throughout the linear protein sequence, they are close to each other in the active 3-dimensional conformation. Chymotrypsin, which is a major serine protease, is found in helminths. Chymotrypsin can be divided into 3 main subfamilies based on its substrate specificity; trypsin-like, chymotrypsin-like, and elastase-like. The proteases in these 3 subfamilies share a similar tertiary structure, but their substrate cleavage specificities differ; trypsin-like, in which a cleavage of amide substrates follows Arg or Lys at the P1 position (Fig. 1A); chymotrypsin-like, in which a cleavage occurs following 1 of the hydrophobic amino acids at P1 (Fig. 1B); and elastase-like, in which a cleavage follows an Ala at P1 (Fig. 1C).
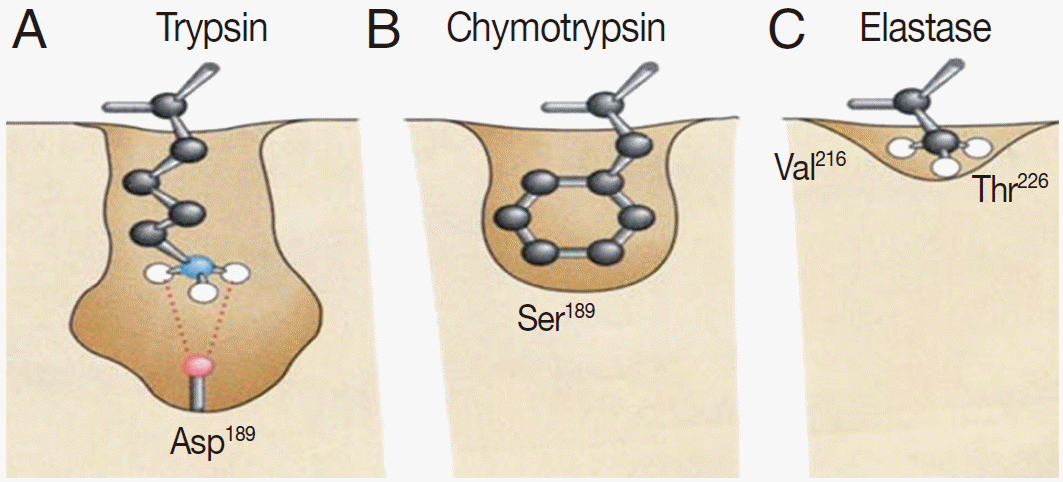
The pattern and characterization of the binding pocket responsible for specificity of serine proteases. (A) Trypsin specificity is due to a negatively charged aspartic acid (Asp) located in the base of the binding pocket. Thus, it specifically cleaves peptide bonds of positively charged residues, i.e., lysine (Lys) and arginine (Arg). (B) Chymotrypsin specificity is due to a deep hydrophobic pocket containing serine (Ser) and glycine (Gly). This contributes to specifically cleave peptide bonds of large hydrophobic residues, i.e., phenylalanine (Phe), tryptophan (Trp), and tyrosine (Tyr). (C) Elastase has a much smaller binding pocket containing Arg and Lys than Trypsin or Chymotrypsin and prefers to cleave peptides of small, neutral residues, such as alanine (Ala), glycine (Gly), and valine (Val).
These enzymes are usually synthesized as inactive precursor zymogens, which are converted to the smaller activated enzymes by cleavage processes involving a conformational change. Conformational change is necessary for hydrolytic activity. There are 4 steps involved in the chymotrypsin catalysis mechanism, which include substrate binding, nucleophilic attack, protonation, and deacylation. A variety of structural features are responsible for the catalytic effectiveness of these enzymes [4] (Fig. 2A-D).
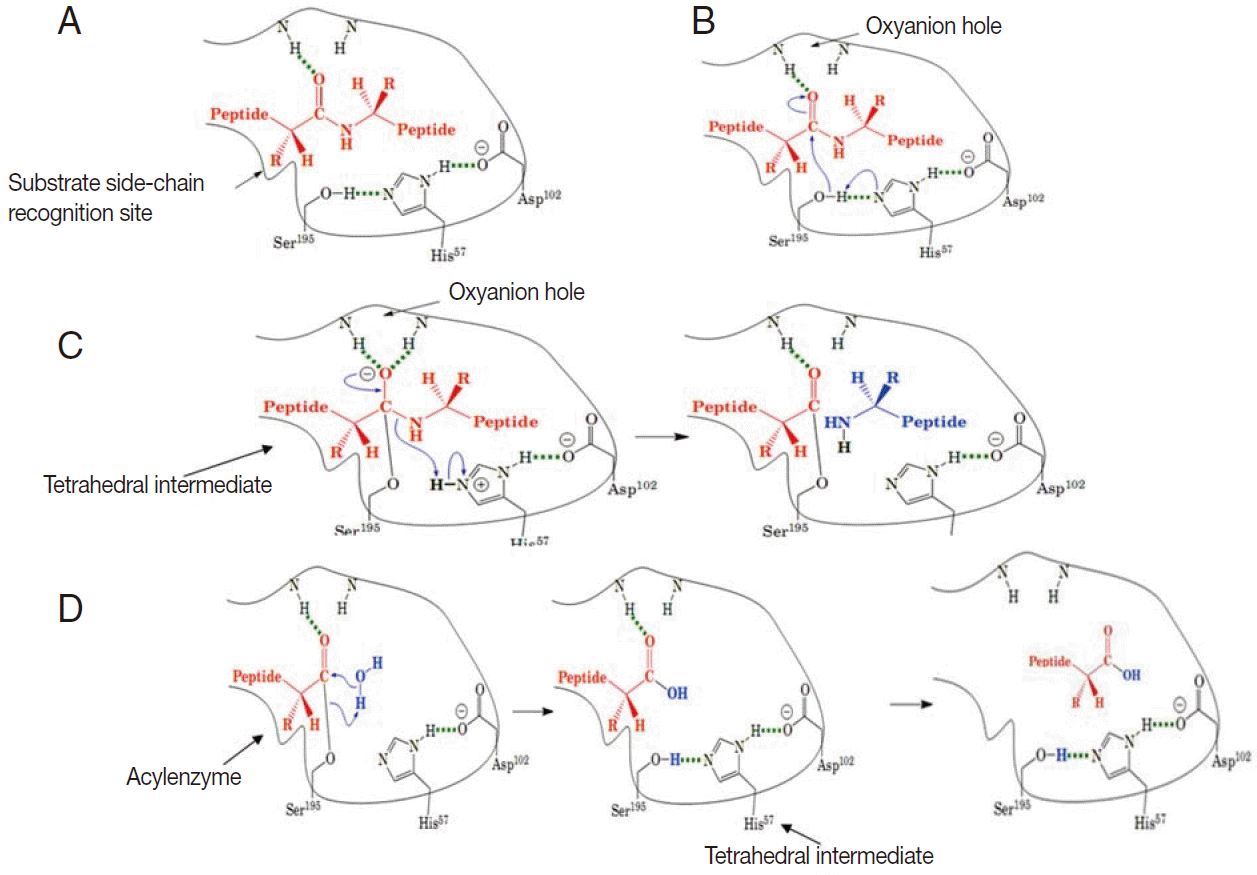
A schematic illustration of general catalytic mechanism for serine proteases (modified from Mark Brandt, 2001). (A) Substrate binding: substrate binds to the recognition site of the serine protease and exposes the carbonyl of the scissile amide bond. (B) Nucleophilic attack: His 57 attracts the proton from the hydroxyl group of Ser 195 and the Sser 195 attacks the carbonyl of the peptide substrate. (C) Protonation: The amide of peptide subtract accepts a proton from His 57 and dissociates. (D) Deacylation: water molecule attacks the acyl-enzyme complex and catalytic triad is restored.
Subtilisin is another family of serine proteases and was also first found in prokaryotes. Subtilisin has a completely different protein structure, but it has the same catalytic residues and shares the same catalytic mechanism that utilizes a catalytic triad.
HELMINTH SERINE PROTEASES
A summary of serine proteases identified in parasitic nematodes, cestodes, and trematodes are presented in Tables 1 and 2, and their individual characteristics are described as follows.
PARASITIC NEMATODE SERINE PROTEASES
Serine proteases from Trichinella
Trichinella is an intracellular nematode that infects a wide variety of animals. The complete life cycle of the parasite is completed in a single host via the invasion of intestinal epithelial and skeletal muscle cells. A recent study of proteases throughout the life of Trichinella spiralis found that excretion-secretion (ES) and crude extracts of muscle stage larvae show substantial serine protease activity against structural proteins, whereas newborn larvae and adult worms principally degrade hematic proteins. This stage-specific proteolytic activity contributes to the breakdown of both mechanical and humoral barriers within the host during parasite infection. These serine proteases are targets of the antibody response, which can inhibit the protease activity and possibly contribute to the impairment of the parasite in a sensitized host [5,6].
During the invasion of epithelial cells, the larvae released several glycoproteins that bear the highly antigenic sugar moiety, tyvelose (3, 6-dideoxy arabinohexose). Monoclonal antibodies against tyvelose protect against infection, which implicates that tyvelose-bearing glycoproteins play keys roles in intestinal epithelium invasion and niche establishment. With the aim of investigating these glycoproteins at the molecular level, Romaris et al. [7] first isolated glycoproteins by affinity chromatography technique using monoclonal antibodies (mAbs). De novo peptide sequencing combined with cDNA library screening identified that these glycoproteins are serine proteases (TspSP-1). Western blot analysis and immunohistochemistry indicated that these glycoproteins are muscle larvae (ML) stage specific and are synthesized in α stichocytes. Furthermore, the inhibition of epithelial cell invasion and migration by mAbs against TspSP-1 indicated that TspSP-1 could play an important role in degrading cytoplasmic or intercellular proteins, thereby facilitating the movement of the larvae [7]. Subsequently, Nagano et al. [8] also isolated a serine protease, named Ts23-2, from a cDNA library of T. spiralis muscle larvae. The Ts23-2 gene is only transcribed after the completion of cyst formation. The protease activity of the recombinant catalytic domain was confirmed using synthetic peptide substrates, indicating that it is a plasmin-like protease [8].
Recently, another member of this subfamily, named TspSP-1.2, was characterized. The anti-serum against TspSP-1.2 can partially prevent the larval invasion of intestinal epithelial cells. Furthermore, the recombinant TspSP-1.2 protein induced a partial protective immunity in mice. These results indicated that TspSP-1.2 contributes to the larval invasion of host intestinal epithelial cells and could be a potential vaccine candidate against T. spiralis infection [9]. A similar protein (TppSP-1) from Trichinella pseudospiralis muscle larvae was identified by Cwiklinski et al. [10]. Analysis of the deduced amino acid sequence found that the histidine residue of the catalytic triad in TsSP-1 was replaced with an arginine residue in the TppSP-1. This could lead to the loss of proteolytic activity, and the role in the T. pseudospiralis-host interaction needs further research [10]. Trap et al. [11] identified another putative serine protease by screening a library from T. spiralis adult-newborn larvae mixed stage with a radioisotope-labelled DNA probe. TsSerP contains 2 trypsin-like serine protease domains flanking a hydrophilic domain. Northern blot analysis of the expression profile for TsSerP genes demonstrated that it was expressed in all life cycle stages of the parasite. Western blot analysis using soluble and E-S antigens found that it was not detected in ES products. Immunolocalization showed that TsSerP is expressed on the peripheral regions and the esophagus of T. spiralis muscle larvae and adult worms. Thus, TsSerP may be involved in the parasite’s moulting process and digestive function [11].
Liu et al. [12] identified a newborn larval stage-specific serine protease gene (NBL1) via a subtractive cDNA library of T. spiralis newborn larvae. It includes 2 regions, a catalytic domain and a C-terminal domain. Epitope mapping using truncated variants of rNBL1 indicated that the C-terminal part of NBL1 is the main immunodominant region. NBL1 showed encouraging potential in the early detection of Trichinella infection and protective immunity against T. spiralis infection in pigs [13]. Based on the high immunogenicity of the C-terminal domain, we hypothesized that during the newborn larval invasion of the host, it may divert the immune response away from the functional regions of NBL1 to contribute to host invasion.
The multiple serine proteases identified at different stages of T. spiralis indicated the existence of a superfamily of serine proteases in T. spiralis. Each member of serine protease families may have different functions in parasite infection, which depends on stage-specific expression, location, and the presence of a regulation domain of the serine protease. Further studies are required to fully understand their functions in parasite-host interplay.
Serine proteases from Trichuris muris
T. muris is a parasitic nematode of mice in which an infective larva invades host intestinal mucosa and develops into an adult worm. The anterior portion of an adult worm embeds within a syncytial tunnel derived from host cecal epithelium. There are 2 major serine peptidases with specific activity for collagen-like molecules in the ES antigens of T. muris adult worms. Interestingly, the activity of both serine peptidases was not observed in worm extract, which suggests that the enzymes are present in the adult worm as inactive precursors or inactivated by endogenous peptidase inhibitors. The ability for degradation of the basement membrane by live worms suggested that these peptidases could be involved in the invasive process. These peptidases also could play important roles in the production and subsequent maintenance of the parasites' syncytial habitat. Moreover, authors speculated that they could contribute to the pathology of trichuriasis by disrupting the integrity of epithelial cell membranes [14].
The intestinal mucus barrier plays a significant role in the expulsion of gastrointestinal nematodes. The mucin is pivotal during the formation of the mucus layer. A recent study found that serine proteases secreted by T. muris can degrade the major intestinal mucin Muc2 and depolymerize the mucin network. Thus, it was suggested that serine proteases secreted by T. muris could contribute to the modification of the parasitic niche to prevent clearance from the host or to facilitate efficient mating and egg laying [15]. Given its essential role in the intestinal stage, this serine protease could be an attractive drug target and should be characterized at the molecular level.
Serine proteases from Anisakis simplex
Anisakiasis is a gastrointestinal tract disease, which is caused by the consumption of raw or undercooked seafood that contains larvae of the nematode A. simplex. After ingestion, A. simplex larvae penetrate the mucosa, submucosa, and muscularis of the host stomach and intestine and may migrate to the omentum, liver, pancreas, or gall bladder. Sakanari and McKerrow [16] found that the secretion of infective larvae contains trypsin-like serine proteases that degrade connective tissue extracellular matrix (ECM). Judy et al. [17] isolated 4 serine protease genes of A. simplex using degenerate oligonucleotide probes based on the consensus regions of mammalian serine proteases. One of these genes is 67% identical to the rat trypsin II gene. Alignment of these 2 genes revealed that the intron-exon junctions are conserved between nematodes and rats, confirming the structural and functional similarity of the 2 genes. Thus, serine proteases of infective larvae may be involved in the degradation or digestion of host tissue [17].
Meanwhile, Stephen et al. [18] purified 2 serine proteases from the infective larvae of A. simplex using different affinity chromatography approaches. The 26 kDa-protease is similar to the trypsin of the domestic pig, Sus scrofa. The second serine protease was similar to the extracellular serine protease of the pathogenic bacterium Dichelobacter nodosus, which can degrade elastin, keratin, and collagen. The mechanism involved in the tissue destruction caused by the 2 serine proteases is far from being decrypted and needs further research [18].
Serine proteases from Ascaris suum
A. suum, also known as the large roundworm of pigs, is an intestinal nematode. During the process of A. suum sperm activation, sperm differentiates from immature spermatids into mature and motile spermatozoa. Recently, study of sperm activation indicated that serine protease is responsible for A. suum sperm activation. This serine protease was purified and identified by de novo sequencing. The purified protease showed strong activity in sperm activation, and it can be inhibited by the serine protease inhibitor PMSF. Finally, the full length cDNA named As-TRY-5 was cloned by RACE-PCR using the degenerative primers based on the peptide sequence. Sequence comparisons indicated that As-TRY-5 shares a high degree of homology with trypsin-like serine proteases of eukaryotes. A further study using a serine protease inhibitor (As-SRP-1) that was released by the activated sperm indicated that during the spermatogenesis process, the activity of As-TRY-5 was regulated by this serine protease inhibitor. This could be significant during postcopulatory sexual selection [19].
Serine proteases from filarial worms
Onchocerca volvulus is an important filarial nematode that causes subcutaneous filariasis of humans and affects the eyes and skin. The infective larvae, male worms, and microfilariae migrate through the host tissue. A proteolytic activity study indicated that there is a 40 kDa neutral elastase in ES products of microfilariae, which can degrade components of the dermal extracellular matrix, collagen type IV, fibronectin, and laminin but cannot degrade intact immunoglobulins. Based on this proteolytic activity, authors suggested that the elastase of microfilariae plays an important role in the degradation of elastic fibres of the host tissue. Moreover, the elastase proteolytic activity is also present in males, but absent in ES products of females. This is correlated with the different behavior of adult worms; the adult males are able to migrate from 1 nodule to another, whereas adult females only reside in nodules [20]. In addition, a stage-specific 43-kDa serine elastase was also found in infective larvae of Onchocerca lienalis. The specific elastase of L3 larvae most likely contributes to L3 migration from the blackfly bite site to different tissues of the body where the adults will develop and form nodules [21]. Blisterase is a subtilisin-like serine protease and plays important roles in nematode biology including the cuticle production and maintenance, neural signalling, and nematode development. Thus, it is a potential drug target for controlling parasite infection. Catherine et al. [22] isolated blisterase from a cDNA library of the infective larvae (L3) of O. volvulus. A fragment of blisterase was cloned and expressed in insect cells with maximal activity at a neutral pH. However, the roles of the blisterase in the O. volvulus-host interaction remain unknown [22].
Complement plays multiple roles in both innate and adaptive immunity, such as mediating the adherence of myeloid cells to the parasite and subsequently killing parasite and directing cellular recruitment. Rees-Roberts et al. [23] reported that secreted products of Brugia malayi microfilariae can cleave the C5a and completely abolish C5a-mediated chemotaxis of human peripheral blood granulocytes. The inhibition was blocked by a serine protease inhibitor, indicating 1 or more types of serine proteases are responsible for the cleavage of C5a. It has been speculated that serine proteases from B. malayi may suppress the immune system and induce immune tolerance, hindering parasite elimination [23].
Serine proteases from Ancylostoma caninum
Hookworm disease results from infection by a hematophagous nematode of the genus Ancylostoma that lives in the small intestine of the host. Now, more than 1 billion people are infected with this parasite worldwide. Hookworms cause anemia by extracting host blood from lacerated capillaries in the mucosa of the small intestine over an extended period of time. Peter et al. [24] found a 36 kDa elastolytic enzyme with anti-clotting properties in ES products of third-stage infective filariform larvae of Ancylostoma caninum. This elastolytic enzyme interferes with fibrin clot formation and promotes fibrin clot dissolution. The protease can degrade fibrinogen into 5 smaller polypeptides with anticoagulant properties. In addition, this protease can convert plasminogen to a mini-plasminogen-like molecule. This molecule is analogous to leukocyte elastase and could be related to the specific antihemostatic mechanism of the hookworm. According to their results, authors hypothesized that the parasite uses this enzyme to feed on the villous capillaries by preventing the blood from clotting. Thus, this protease is a potential target for chemotherapeutic or immunological intervention [24].
Serine proteases from Steinernema carpocapsae
S. carpocapsae is a parasitic nematode of insects and is used as a biological control agent to kill several insect pests and vectors. The infective juvenile can enter the host by mouth or anus and invade the hemocoelium. Invasion has been described as a key factor in nematode virulence and is mediated by proteases. Recently, 2 novel serine protease cDNAs (sc-sp-1 and sc-sp-3) from the parasitic stage were isolated by a degenerate RT-PCR based on conserved motifs near the catalytic histidine of serine protease. The sc-sp-1 expression time frame analysis showed that the sc-sp-1 stage-specifically expressed at parasitic stages. It is mainly expressed in the midgut of the insect (L3), where the nematodes will prepare to invade the insect hemocoelium. Further, analysis of the influence of insect tissue on sc-sp-1 expression showed that different tissues of the insect can induce the expression of sc-sp-1 at different times. This could contribute to the parasite’s ability to sense insect tissues at different time points. The peritrophic membrane of the gut wall and the basal lamina are major barriers of host tissue invasion. The study showed that the sc-sp-1 was highly efficient at destroying the peritrophic membranes and caused epithelium cell detachment from the basal lamina. Thus, the function of sc-sp-1 could be the invasion of hemocoelium through the disruption of the midgut barrier [25]. The sc-sp-3 is a multifunctional chymotrypsin-like protease. It not only shares similar biochemical characteristics with sc-sp-1 but also induces caspase-dependent apoptosis in Sf9 insect cells [26]. Recently, a stage-specific elastase-like serine protease gene (Sc-ELA) was isolated by the suppression subtractive hybridization method during the parasitic stage. Sequence comparison and evolutionary marker analysis revealed that Sc-ELA was a member of the elastase serine protease family with potential degradative, developmental, and fibrinolytic activities [27].
In addition to these serine proteases, Balasubranian et al. [28,29] purified 2 insect immune depression-related serine proteases from the ES products of infective-stage S. carpocapsae. Melanotic encapsulation that is formed by the deposition of multiple layers of hemocytes and⁄or melanin is an important insect defence mechanism against parasites. The trypsin-like serine protease and chymotrypsin-like serine protease can prevent melanotic encapsulation by suppressing prophenoloxidase activity or by disrupting the insect hemocyte F-actin cytoskeleton. Although this experimental evidence did not fully elucidate the exact biological roles of the serine proteases during host immune suppression, it contributes to the understanding of the pathogenesis strategy used by S. carpocapsae. Further biochemical and molecular characterization of Sc-Trypsin and Sc-chymotrypsin is required for a complete delineation of their possible functions in helping parasites to infect and survive within the host [28,29].
CESTODE SERINE PROTEASES
Cestodes reside in the digestive tract of their host as adults. However, the larvae are involved in tissue invasion and can migrate into some visceral organs and the central nervous system, causing a range of serious diseases such as sparganosis, echinococcosis, and neurocysticercosis. Some serine proteases involved in host tissue invasion and immune evasion have been characterized in cestodes.
Sparganosis caused by the plerocercoid larvae of Spirometra mansoni usually results from ingesting contaminated food or water. The parasite can migrate to any part of the body, but it usually resides in the skin where it develops into a nodule. Kong et al. [30] purified 3 neutral serine proteases from the extracts of the plerocercoids. Analysis of proteolytic activities showed that 2 trypsin-like proteases of 198 and 104 kDa have collagenolytic activities; however, the 36 kDa chymotrypsin-like serine protease prefers to cleave human recombinant interferon-g and bovine myelin basic protein. In addition, all purified proteins elicited strong antibody responses in infected patients, suggesting that they could be potential antigens in serologic diagnosis of human sparganosis [30].
Cystic echinococcosis (CE), caused by Echinococcus granulosus has a public health importance not only in areas of endemicity but also in countries or regions where the migration of infected people and exchanges of livestock occurs. Antigen 5 (Ag5) is a major secreted component of the larvae of E. granulosus. It has been used as a diagnostic antigen for detection of echinococcosis in humans for many years. To characterize the biological function, Lorenzo et al. [31] isolated the Ag5 gene by RT-PCR on the basis of the amino acid sequences of tryptic fragments. Analysis of the nucleotide sequence indicated that Ag5 is synthesized as a single polypeptide chain, which afterwards is processed into 2 subunits. The 22-kDa subunit contains a highly conserved glycosaminoglycan-binding motif. This motif may help confine Ag5 to the host tissue surrounding the parasite. The amino acid sequence of the 38 kDa subunit shows high similarity to serine proteases of the trypsin family, specifically to the neutral proteases of mast cells. However, the catalytic serine residue is replaced by threonine. The biochemical characterization of Ag5 showed that neither proteolytic activity nor binding to protease inhibitors could be found in native purified Ag5. This intriguing feature of Ag5 suggests that it could possess a highly specific substrate or a specific activation step to carry out new biological function [31].
Furthermore, immunolabelling with specific antibodies against rAg5 showed that Ag5 is strongly expressed in the tegument of the protoscolex and the embryonic membrane of the egg as well as on the surface of the oncosphere. Meanwhile, it is also weakly expressed in the tegument of the adult. Nevertheless, the roles of Ag5 remain unknown, but the expression in all stages of the life cycle confirms that Ag5 is a potential antigen for use in diagnosis and vaccine development in both intermediate and definitive hosts [32].
In a recent study, a trypsin-like serine protease of Taenia solium cysticercus termed TsAg5 was identified. It is the first serine protease gene characterized from T. solium so far, and it is highly homologous to E. granulosus antigen Ag5. Western blot analysis showed that TsAg5 can be detected in the cyst fluid and ES antigens of the cysticercus. The recombinant trypsin-like domain of TsAg5 showed trypsin-like activity and can be inhibited with chymostatin. Furthermore, evaluation of the diagnostic potential of this domain in detecting human cysticercosis by immunoblot assay showed that the trypsin-like domain was moderately sensitive and specific for neurocysticercosis [33].
Schistocephalus solidus is a tapeworm that infects fish. A 23.5-kDa chymotrypsin-like serine protease with collagenolytic activity was identified in the extracts of procercoids. However, it was absent in plerocercoids and adults. The specific expression of the chymotrypsin-like serine protease in procercoids may be necessary for procercoid invasion via the penetration of the host's intestinal wall [34].
Although increasingly fruitful reports on serine proteases suggest their importance in cestode infections, they still remain to be extensively characterized and assessed for their therapeutic values.
TREMATODE SERINE PROTEASES
Serine proteases from the liver fluke
Fasciola hepatica and F. gigantica are the parasites that cause liver fluke disease (fascioliasis). It is not only an important human disease but it also affects cattle and sheep. Infection causes worldwide economic losses of approximately 2 billion dollars per year. Carmona et al. [35] purified a secreted dipeptidylpeptidase (DPP) from F. hepatica by gel-filtration and ion-exchange chromatography. It was found to be a serine protease that is expressed in newly excysted juvenile, immature, and mature flukes. Authors suggested that the parasite DPP may function in the digestion of host macromolecules into peptides. These peptides could be absorbed as nutrients by the parasite’s intestine, which could profit the parasite [35]. In a recent study, a 60-kDa neutral serine protease designated as serine PIc was separated from F. gigantica. A study of biological characteristics found that the activity and stability of the serine protease depended on divalent cations and temperature. Enzyme activity assays indicated that proteolytic activity increased followed by the development of F. gigantica, which suggests that it has a very important physiological role, but the precise function remains unknown [36].
Serine proteases from schistosomes
Schistosomiasis is a serious human disease in the tropics, which affects millions of people. Infection of humans by Schistosoma mansoni begins following the invasion of intact skin by the cercariae. The penetration of the skin is facilitated by secretions from the acetabular and head glands. Disruption of these potential mechanisms by specific drugs/vaccines may provide therapeutic benefits. Thus far, a number of studies have confirmed that cercarial elastase (SmCE), which has a chymotrypsin-like activity, is a major histolytic protease involved in skin invasion. Northern blot analysis indicated that it is stage-specific and is only expressed in cercariae. Further anatomical location of SmCE mRNA in tissue sections of developing larvae showed that it is only synthesized in acetabular gland cells of developing cercariae. This further indicated that SmCE is regulated within a limited developmental frame in a specialized cell [37]. A subsequence protease activities assay indicated that a serine protease with “trypsin-like” activities from secretions of cercariae could also be involved in host invasion [38]. To evaluate the relative roles of these 2 serine proteases in larvae invasion, both of 2 serine proteases were analyzed by southern blot, genomic PCR, and immunolocalization. These results demonstrated that only single SmCE with activities against macromolecular substrates is responsible for human skin invasion, and serine protease with trypsin-like activities is a contaminant from the intermediate host snail [39].
To date, 8 isoforms of S. mansoni stage-specific elastases have been identified based on amino acid and promoter sequence homology. In addition, investigation of SmCE ortholog genes in the related species Schistosoma haematobium and S. douthitti found that multiple CE isoforms exist in both species [40]. By contrast, in another schistosome species, Schistosoma japonicum, no SmCE ortholog was identified. This result indicates that the expansion of the cercarial elastase genes is limited to the human-specific schistosomes [41,42]. To further explore the roles of SmCE gene expansion in S. mansoni, James et al. [43] investigated the profile of transcript and protein expression patterns and substrate preferences of the expanded SmCE gene family. The results revealed that these SmcE isoforms are similarly expressed throughout the S. mansoni life cycle. They have largely similar substrate specificities. According to these results, authors suggested that the majority of protease isoforms share a conserved function for a common pool of substrates [43]. Thus, the expansion of the SmCE gene family is functionally redundant and is a direct increase in the amount of protease. In addition, activity-based profiling showed that the activity of SmCE also presented in 6-week daughter sporocysts, suggesting that 1 or more of the SmCE isoforms could have a novel role in its intermediate host [43].
Proteases have long been hypothesized as aiding parasites in evading the immune response of the host by degrading immune effectors or modulating the cellular immune response. Studies have shown a direct correlation between levels of specific IgE and protective immunity against schistosomes in humans [44,45]. Analysis of the immune evasion ability of parasites showed that extracts from cercarial and schistosomular stages of S. mansoni can cleave human, mouse, and rat IgE, but not human IgA1, IgA2, or IgG1. This cleavage can be inhibited by serine protease inhibitors. This indicates that during the establishment of mature infections, an elastase-like serine protease helps the parasite to evade IgE-mediated protective reactions [46]. A recent study using a highly purified SmCE found that these SmCE cleave IgE at solvent-exposed interdomain regions of the IgE-Fc. This sequence of cleavage is also present in numerous key molecules involved in regulating immunity, including FcγRI, IL-2, IL-10R, IL-12R, and TLR3. Thus, additional studies are required to determine more genuine substrates for SmCE, which will help us to completely understand the roles of SmCE in immune evasion [47].
To confirm such a role in vivo would require analysis of the immune clearance of parasites in a living host following chemical or genetic knockout of the protease. Because the degradation of immune effectors is vital for parasite immune evasion, the inhibition of this degradation pathway offers a valid approach for developing novel chemotherapeutic agents. Thus, further investigation to determine serine protease biological function and the evaluation of its potential as a drug target are needed.
CONCLUSIONS AND PERSPECTIVES
Worm disease remains a major neglected disease of humanity in many regions, especially in developing countries. Because there is an emerging drug resistance, and there is an inability of current drugs to prevent reinfection, the identification of novel chemotherapeutic agents and vaccines for protection from helminth pathogens is a public health priority. The challenge of developing new therapies involves several steps, the first of which is to identify and characterize potential targets of drug or vaccine treatments. This review presents evidence that serine proteases do not only have important functions in the regulation of endogenous physiological processes of parasitic helminths, but are also actively involved in host-parasite interactions. These findings will undoubtedly make serine proteases to be an exciting field of helminth research. The newly available genome sequences of some helminths combined with large EST libraries should facilitate future work in this area enormously; for example, a bioinformatic analysis of the genome sequence dataset along with proteomic and microarray data will accelerate the identification of more serine proteases. Useful tools to characterize helminth serine protease function such as development regulation, fertilization, invasion of host tissues, and immune evasion include crystallography for the determination of the 3D structure, RNA interference for the silencing of gene expression, and monoclonal antibodies for the inhibition of protease activity as well as colocalization studies to validate an association between a particular serine protease and a putative substrate.
This comprehensive analysis not only expands the growing knowledge base regarding helminth serine proteases but also provides a platform for the exploration of their biological functions and potential as targets of effective chemotherapeutic or immunological treatments. Anthelmintic drugs found via high throughput screening for small molecule inhibitors of some of the critical serine proteases involved in the host-parasite interplay will become a practical reality in the near future. Moreover, anthelmintic drug discovery needs to take into accounts not only the target enzymes within the parasite but also similar enzymes in the host because inhibition of host enzymes can result in toxicity to the host. Thus, the increase in information that is becoming available on the serine proteases of humans is also beneficial to parasitologists. In addition, substrate identification will also undoubtedly yield insight into many different areas of helminth biology. The fine specificity of the relationships between serine proteases and their substrate proteins could provide a new molecular paradigm for understanding host-parasite co-evolution.
Acknowledgements
We thank the Med-Vet-Net EU contract (WP 27 TrichiMED), Chinese Academy of Agricultural Sciences Engineering Innovation fund (20140204066NY), ANR TrichiVac contract, and China scholarship council for financial support.
Notes
We have no conflict of interest related to this work.

