Comorbid Gastric Adenocarcinoma and Gastric and Duodenal Strongyloides stercoralis Infection: A Case Report
Article information
Abstract
Strongyloides stercoralis can cause systemic infection, termed strongyloidiasis, and gastrointestinal ulcer disease in immunocompromised patients. However, to our knowledge, there are no reported cases of comorbid gastric adenocarcinoma and S. stercoralis infection. Here, we report a case of an 81-year-old Korean man who presented with S. stercoralis infection coexisting with early gastric adenocarcinoma (T1aN0M0). S. stercoralis eggs, rhabditiform larvae, and adult females were observed in normal gastric and duodenal crypts. They were also observed in atypical glands representative of adenocarcinoma and adenoma. Preliminary laboratory tests revealed mild neutrophilic and eosinophilic leukocytosis. A routine stool test failed to detect rhabditiform larvae in the patient’s fecal sample; however, S. stercoralis was identified by PCR amplification and 18S rRNA sequencing using genomic DNA extracted from formalin-fixed paraffin-embedded tissues. Postoperatively, the patient had a persistent fever and was treated with albendazole for 7 days, which alleviated the fever. The patient was followed-up by monitoring and laboratory testing for 4 months postoperatively, and no abnormalities were observed thus far. The fact that S. stercoralis infection may be fatal in immunocompromised patients should be kept in mind when assessing high-risk patients.
INTRODUCTION
Despite the advances in hygiene processes, approximately 30-100 million people in tropical and subtropical regions are infected with Strongyloides stercoralis [1,2]. S. stercoralis is well known to be able to induce systemic infection (strongyloidiasis) and/or gastrointestinal ulcer disease in immunocompromised patients, which could further increase patient mortality [1,3]. However, to the best of our knowledge, there are no reported cases of comorbid gastric adenocarcinoma and S. stercoralis infection. Here, we present a case of gastric adenocarcinoma comorbid with S. stercoralis infection in an elderly man with a long history of diabetes mellitus.
CASE RECORD
An 81-year-old Korean man presented with weight loss, poor oral intake, and abdominal discomfort for 3 months in November 2013. Although the patient had hypertension and diabetes mellitus for several years and had been treated with standard medication, he was relatively healthy until recently. Physical examination showed no signs of fever, chills, diarrhea, abnormal bowel movement or sound, or skin rash. Furthermore, the patient did not report any previous intake of alcohol, tobacco, herb mediation, or inhaled corticosteroids. In addition, he had no significant family history or recent history of travel to tropical or subtropical regions. Esophagogastroduodenoscopy revealed chronic atrophic gastritis with intestinal metaplasia and multiple erosions in the stomach; in particular, an ulcerative lesion, suggestive of type IIc early gastric cancer, was observed in the anterior wall of the antrum, and an atrophic lesion was observed in the duodenum (Fig. 1). Microscopic examination of a biopsy specimen from the gastric mass in the antrum revealed a well-differentiated adenocarcinoma as well as parasite eggs, rhabditiform larvae, and adult worms presumably of S. stercoralis in normal gastric pits. Scattered eosinophils were not generally observed in the antrum (Fig. 2A); however, some parasite eggs and adult worms were observed along with scattered eosinophils in the mucosa of normal duodenal pits (Fig. 2B).
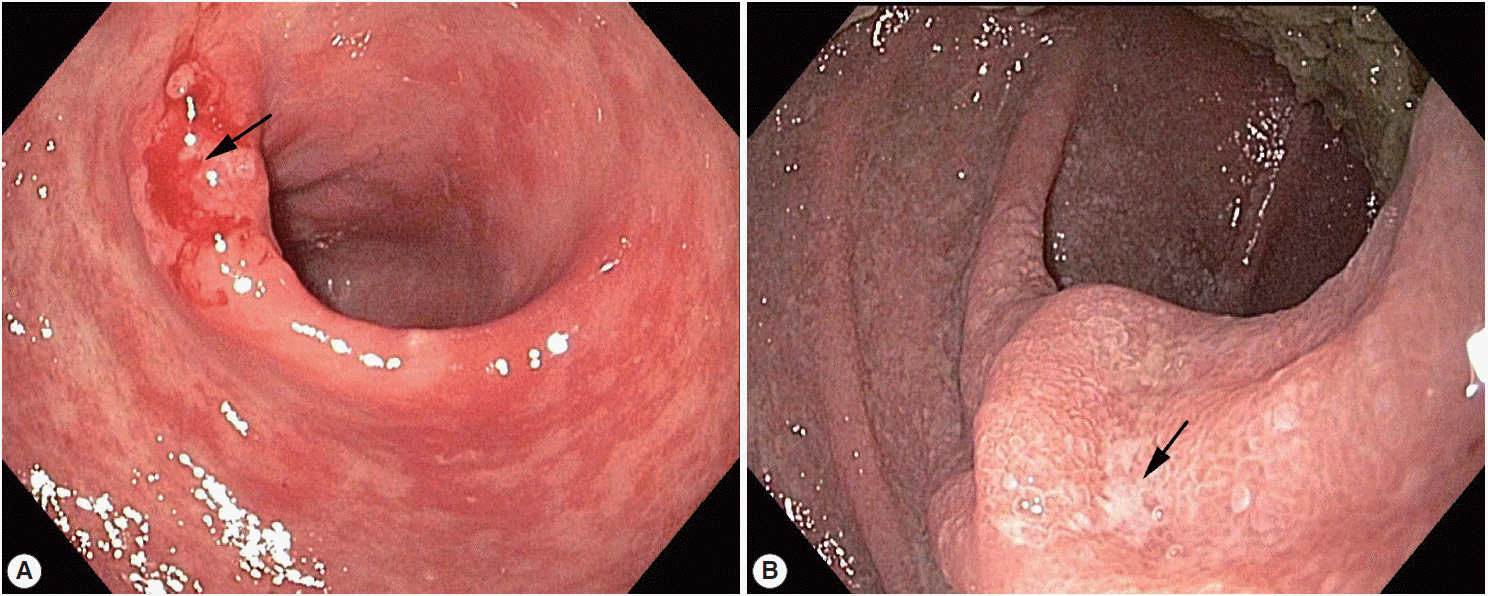
Esophagogastroduodenoscopy showing (A) a superficial mucosal ulcerative lesion suspicious of type 0–IIc early gastric cancer in the anterior wall of the antrum and (B) an atrophic lesion in the duodenum (arrows).
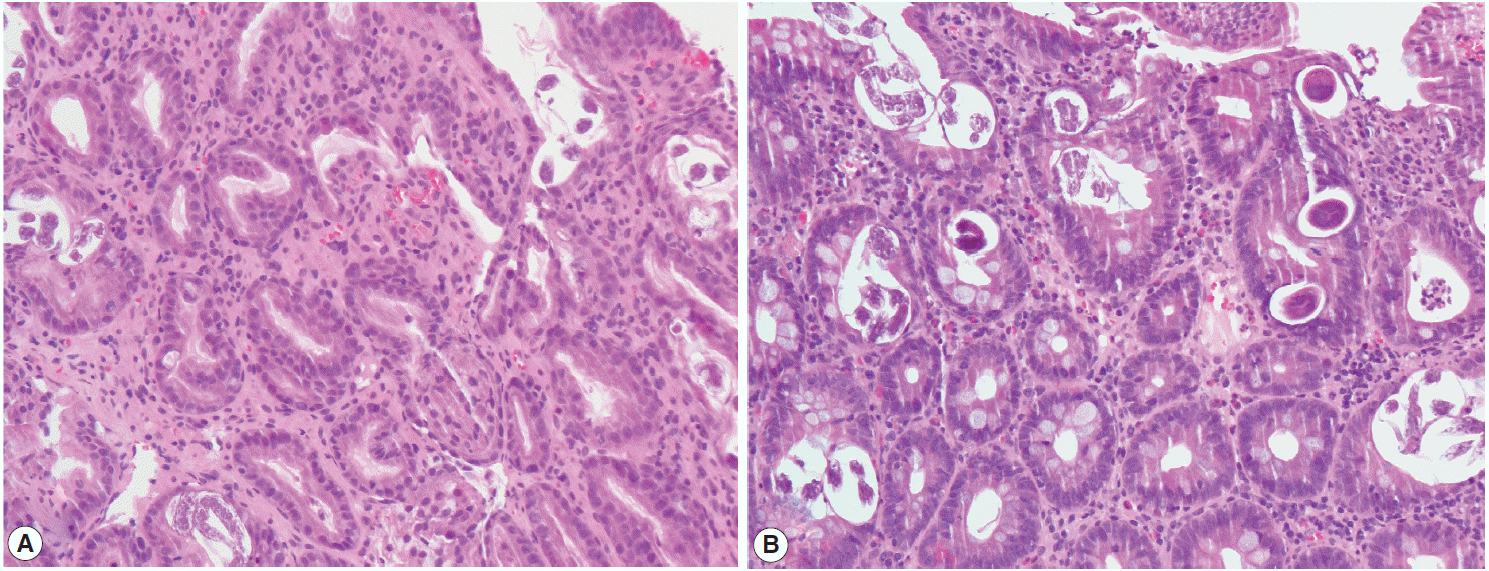
Histology of the biopsied stomach and duodenal tissues demonstrating a parasitic infection. Multiple eggs, adult females, and larvae of Strongyloides stercoralis are seen in the crypts of the stomach (A) and duodenum (B) (×20 objective).
Preliminary laboratory tests detected mild anemia (hemoglobin concentration: 10.7 g/dl, hematocrit: 30.3%), and neutrophilic (8,240/μl) and eosinophilic (550/μl) leukocytosis. The patient had a sodium level of 119 mmol/L, potassium level of 4.2 mmol/L, albumin level of 2.9 g/dl, and prealbumin level of 5.3 mg/dl in serum. An abdominal CT scan indicated suspicious wall thickness in the prepyloric antrum, but no distinct mass-like lesion was observed. After 1 month, the patient underwent subtotal gastrectomy and D2 lymph node dissection. The resected gastric specimen comprised of 2 individual lesions: a moderately differentiated adenocarcinoma of type IIc early gastric cancer (T1aN0M0) and a tubular adenoma. As was the case for the biopsy specimen described above, multiple parasite eggs, rhabditiform larvae, and adult worms presumably of S. stercoralis were found in normal gastric and duodenal gland pits. Moreover, parasite eggs and adult worms were also observed within atypical glands of the gastric adeno carcinoma (Fig. 3A) and adenoma (Fig. 3B), respectively. In addition, a foreign body reaction (Fig. 3C) and scattered eosinophils were observed in the duodenum, and a microabscess was observed in one of the gastric regional lymph nodes (Fig. 3D).
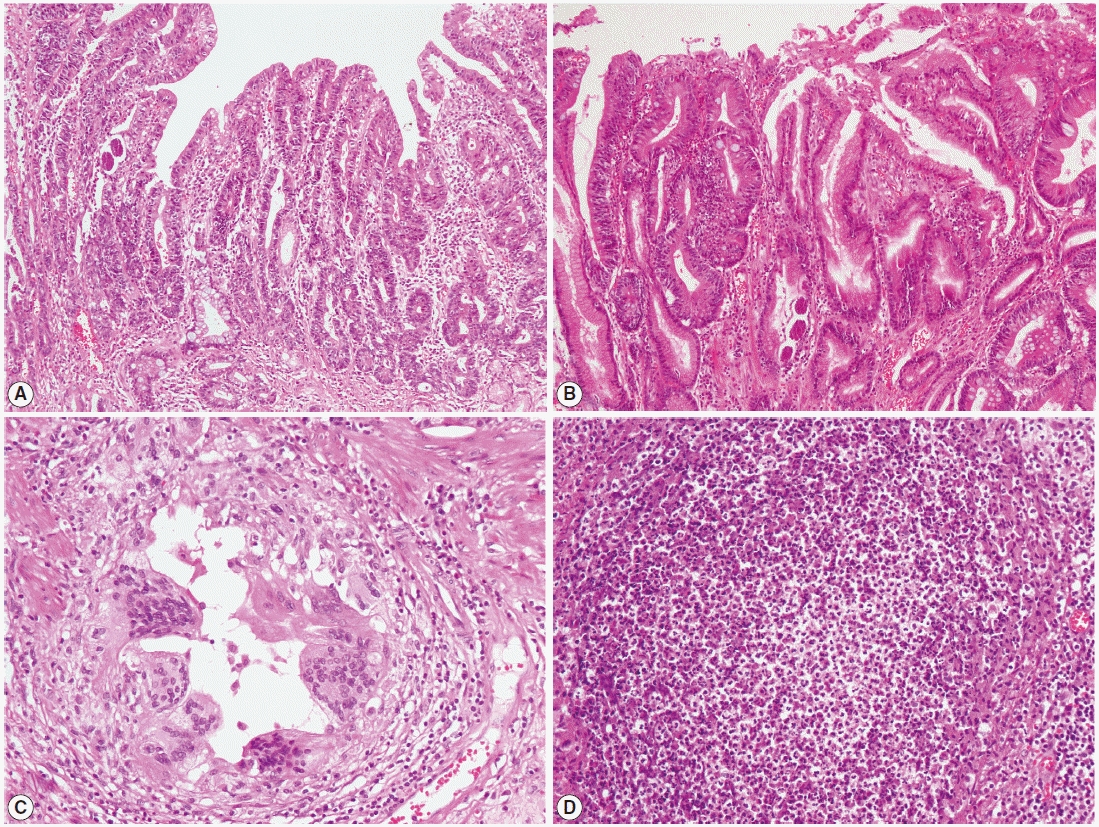
Histologic examination of the resected stomach tissue demonstrating a parasitic infection. (A, B) Eggs and adult females of Strongyloides stercoralis within atypical glands of gastric adenocarcinoma (A) and adenoma (B) (×10 objective). (C, D) Foreign body reaction in the duodenum (C) and a microabscess in one of the gastric regional lymph nodes (D) (×20 objective).
As the routine stool test failed to detect filariform larvae in fecal samples, PCR was performed on DNA samples extracted from a formalin-fixed paraffin-embedded gastric tissue block, aiming to identify S. stercoralis. The following 2 pairs of specific primers used to identify the S. stercoralis 18S rRNA gene have been previously described [4,5]: forward 1, 5´-ATCGTGTCGGTGGATCATTC-3´; reverse 1, 5´-CTATTAGCGCCATTTGCATTC-3´ [4]; forward 2, 5´-GAATTCCAAGTAAACGTAAGTCATTAGC-3´; and reverse 2, 5´-TGCCTCTGGATATTGCTCAGTTC-3´ [5]. A positive band was amplified at 114 and 101 bp for the targeted S. stercoralis gene and at 110 bp for the internal amplification control (Fig. 4). The histopathology and molecular results were consistent with the presence of S. stercoralis within the gastric adenocarcinoma. Following surgical intervention, persistent fever developed. The patient was treated with albendazole (400 mg twice daily) for 7 days; the patient’s fever was subsequently alleviated, his inflammatory marker levels returned to within normal ranges, and he was discharged from the hospital. The patient was followed-up by monitoring and additional laboratory testing for 4 months postoperatively. There have since been no observable abnormalities.
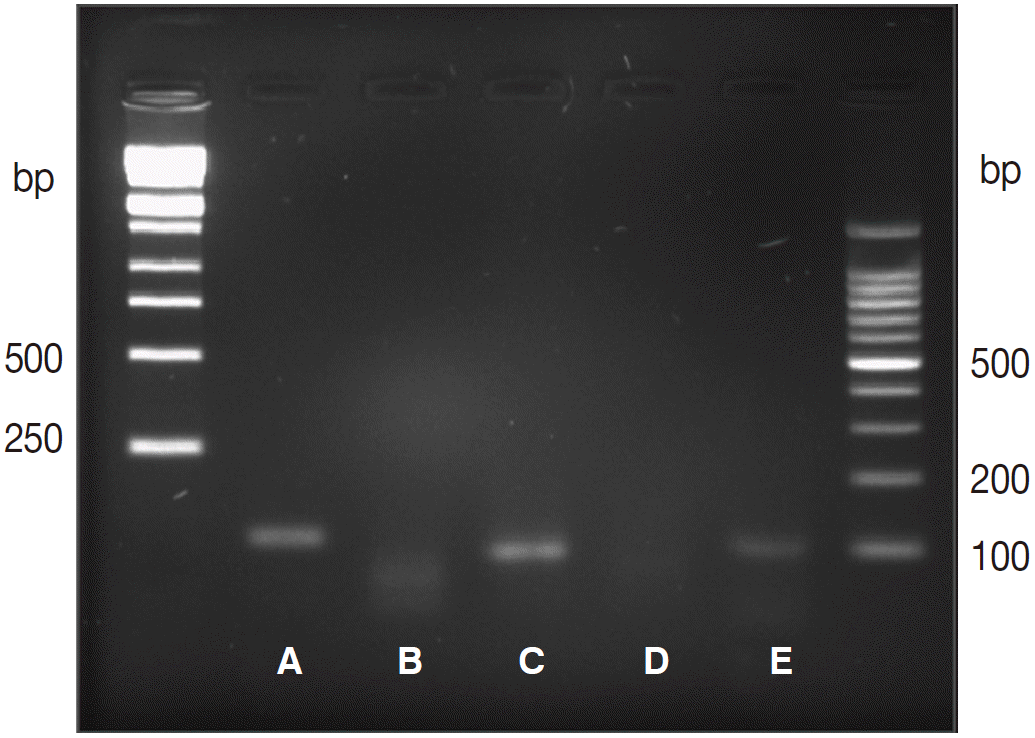
Agarose gel electrophoresis of PCR products amplified using genomic DNA extracted from formalin-fixed, paraffin-embedded gastric tissue, using primers targeting the ribosomal RNA (rRNA) gene. (A) Amplified rRNA gene (114 bp), (B) negative control, (C) amplified rRNA gene (101 bp), (D) negative control, (E) amplified human beta-globulin internal control (110 bp).
DISCUSSION
S. stercoralis is an intestinal nematode parasite that may be able to reproduce in humans [1]. Humans can be infected by third-stage filariform larvae [6,7]. S. stercoralis is transmitted from the soil and penetrates the skin, resulting in an infection. After such exposure, it can be transported to the respiratory system via the bloodstream [1,6,7]. The parasite can then migrate along with swallowed sputum and enter the gastrointestinal tract where it matures, particularly in the duodenum and upper jejunum [6,7]. Internal autoinfection is a major differentiating characteristic of S. stercoralis; it allows this helminth parasite to remain within the human body for years. Several years after exposure, this can contribute to the development of a fatal disease [1]. Although infection may occur in both immunocompetent and immunocompromised individuals, immunocompromised patients in particular may tend to develop disseminated hyperinfection syndrome and septicemia involving multiple organic systems and resulting in potentially life-threatening complications [3]. Therefore, adequate diagnosis and treatment are critical in patients at high risk for complications due to S. stercoralis infection.
Unfortunately, S. stercoralis is difficult to diagnose clinically, and its laboratory diagnosis is commonly confirmed by the detection of filariform larvae in fecal samples. However, routine stool examination using conventional techniques may fail to identify larvae in up to 70% of cases, because the parasite load is very low and larval output is irregular and minimal [1,7]. In the current case, although parasite eggs and adult worms were observed in both the gastric adenocarcinoma and the gastric pits of biopsied and resected gastric specimens, the stool test failed to detect larvae in the patient’s fecal samples. Accordingly, we performed PCR in an effort to detect the presence of S. stercoralis in formalin-fixed paraffin-embedded gastric tissues. This detection method indeed revealed the presence of S. stercoralis.
Eosinophilia is common in patients with strongyloidiasis. However, eosinophil counts are often normal in some immunosuppressed patients with severe strongyloidiasis [7]. Therefore, testing for eosinophilia is unsuitable for screening for this parasitic infection. Moreover, eosinophils are abundant in tissues in this condition [3]. The patient reported here had mild eosinophilia in serum; meanwhile, scattered eosinophils were rarely observed in the stomach but frequently observed in duodenal tissue. In addition, a microabscess was found in a gastric regional lymph node, which is indicative of febrile, disseminated infection. ELISA, a common serology test, was not performed because it exhibits decreased specificity in patients who are already immunosuppressed [8]. Thus, histopathological examination of tissue or aspirated material could aid in determining the definitive diagnosis.
S. stercoralis can inhabit any segment of the gastrointestinal tract, but cases of gastric involvement are rare, because the stomach is not an ideal site [7]. However, reduction of gastric acid secretion could favor infection and stomach involvement. Thus, S. stercoralis infection could lead to gastric ulcers [7]. To the best of our knowledge, the case reported herein is the first case of comorbid gastric adenocarcinoma and S. stercoralis infection in an elderly patient with a long history of diabetes mellitus. We identified parasite eggs and worms within the gastric adenocarcinoma and adenoma and confirmed the presence of S. stercoralis by PCR using samples from the same tissue.
Two recent systematic literature reviews of S. stercoralis infection cases suggest that corticosteroid use is a primary risk factor and can trigger the development of severe strongyloidiasis [8,9]. Unfortunately, data from the literature do not clarify whether strongyloidiasis associated with corticosteroid use depends considerably on the duration and cumulative dosage of the associated treatment [8,9]. Regarding treatment, ivermectin is reported to be the most effective drug for the treatment of strongyloidiasis, but albendazole might be a valid option in many countries with poor availability of ivermectin [8]. However, standard treatments for strongyloidiasis, especially in the presence of hyperinfection or dissemination, remain a debated issue.
In summary, S. stercoralis can result in disseminated hyperinfection syndrome and septicemia that could rapidly become fatal. Therefore, early diagnosis is critical, particularly in patients with risk factors. However, stool examination, which is the conventional method for S. stercoralis detection, has very low sensitivity. Our experience with this case suggests that endoscopic biopsies and PCR are alternative methods for diagnosing strongyloidiasis.
Notes
All authors declare no conflict of interests related to this report.