Relationship between Antibody-Positive Rate against Plasmodium vivax Circumsporozoite Protein and Incidence of Malaria
Article information
Abstract
The relationship between anti-Plasmodium vivax circumsporozoite protein (CSP) antibody levels and the prevalence of malaria in epidemic areas of South Korea was evaluated. Blood samples were collected from inhabitants of Gimpo-si (city), Paju-si, and Yeoncheon-gun (county) in Gyeonggi-do (province), as well as Cheorwon-gun in Gangwon-do from November to December 2004. Microscopic examinations were used to identify malaria parasites. ELISA was used to quantitate anti-circumsporozoite protein (CSP) antibodies against P. vivax. A total of 1,774 blood samples were collected. The overall CSP-ELISA-positive rate was 7.7% (n=139). The annual parasite incidences (APIs) in these areas gradually decreased from 2004 to 2005 (1.09 and 0.80, respectively). The positive rate in Gimpo (10.4%, 44/425) was the highest identified by CSP-ELISA. The highest API was found in Yeoncheon, followed by Cheorwon, Paju, and Gimpo in both years. The positive rates of CSP-ELISA were closely related to the APIs in the study areas. These results suggest that seroepidemiological studies based on CSP may be helpful in estimating the malaria prevalence in certain areas. In addition, this assay can be used to establish and evaluate malaria control and eradication programs in affected areas.
INTRODUCTION
Plasmodium vivax is a causative agent of relapsing benign tertian malaria, the second leading type of human malaria, which afflicts several hundred millions of individuals annually. The disease is a major public health problem in most tropical regions, as well as many temperate countries, including North and South Korea [1]. The first scientific documentation of malaria occurrence in Korea was published in 1913, although malaria had been prevalent throughout the Korean peninsula for several centuries prior [2]. As a result of a national malaria eradication program conducted with the involvement of the World Health Organization, the incidence of vivax malaria in South Korea has rapidly decreased [3,4]. In fact, vivax malaria was considered eradicated in South Korea in the late 1970s until 2 sporadic cases were detected in the 1980s [5]. In 1993, a case was diagnosed among South Korean soldiers serving in Northern Gyeonggi Province [6], and subsequently, 2 infected civilians were reported [7]. Thereafter, many cases have been reported near the demilitarized zone (DMZ), centering on Paju, Yeoncheon, Cheorwon, Gimpo, Ganghwa, Goyang, and Dongducheon. There is now considerable concern that malaria will become re-established in the region and then expand geographically [8].
The malaria research team at the Korea National Institute of Health (KNIH) has developed several diagnostic methods to support pathological examinations. One of these is an antibody detection method using circumsporozoite protein (CSP), which is a sporogony antigen and surface membrane protein that is expressed in all plasmodial sporozoites. CSP has a central immunodominant region consisting of a short tandem repeat of amino acid sequences that contain multiple copies of the immunodominant B-cell epitope [9]. CSP is classified into 2 serotypes, which differ with respect to the sequences at the repeated region of the CSP gene: VK210 [the dominant form consisting of GDRA(D/A)GQPA repeats] and VK247 [the variant form consisting of ANGA(G/D)(N/D)QPG repeats]. It has been established that Korean vivax malaria belongs to the VK210 type; thus, in a previous study, Lee et al. [10] constructed recombinant CSP from a Korean isolate of the VK210 type.
Serological surveys have provided valuable epidemiological information, especially in areas of low endemicity [11]. The rate of parasitemia is the foundation of the classical method used for measuring the prevalence of malaria. However, the incidence of parasitemia alone may fail to adequately describe the epidemiology of malaria in a given population. For example, when the incidence of malaria is low, mass blood surveys do not yield results commensurate with the work involved [12,13]. In this study, anti-P. vivax CSP antibody levels were determined among populations in epidemic areas, namely, Gimpo, Paju, Yeoncheon, and Cheorwon, to evaluate the efficacy of these antigens in determining local malaria prevalence compared to the incidence of malaria patients.
MATERIALS AND METHODS
Study areas and blood sample collection
The study was conducted in Gimpo, Paju, and Yeoncheon of Gyeonggi Province and Cheorwon of Gangwon Province, South Korea, from November to December of 2004. A blood sample was collected from 1,774 individuals and transferred to the KNIH at the Korean Center for Disease Control and Prevention (KCDC). Blood smears were prepared for microscopic examinations. Sera were separated and stored at -20˚C for antibody analysis. Informed consent was obtained from all individuals.
Ethics statement
All participants were informed of the methodology of the study and signed an informed consent form according to ethical standards. The study procedures, potential risks, and benefits were explained to all participants. All adult participants and parents/kin of child participants in a household provided informed consent. Parents that were unwilling to have their children participate were identified, and the data of these children were subsequently excluded, without prejudice, from study surveys. Furthermore, all data were analyzed anonymously; no patient was identified by the name. Ethical approval of the study was obtained from the KNIH. This study was conducted according to the principles expressed in the Declaration of Helsinki.
Microscopic examinations
Thin blood films were prepared to determine the infectivity of blood samples. The blood films were fixed with methanol and stained with Giemsa solution diluted with buffered water at pH 7.2 to emphasize the parasite inclusions in red blood cells (RBCs). The fixed monolayer of RBCs in this procedure facilitates morphological identification of the parasite to the species level and provides greater specificity than that obtained with thick-film examinations. Thin blood films are often preferred for routine estimations of parasitemia because the organisms are easier to see and count with this method [14]. To estimate the densities of blood-stage parasites by microscopy, the number of asexual parasites observed relative to 200 white blood cells (WBCs) was calculated and then the parasite to WBC ratio was multiplied by 8,000, which is the assumed number of WBCs per microliter of blood [15].
ELISA
ELISA was used to determine whether the blood samples contained antibodies against the CSP VK210 antigens of P. vivax [10]. Briefly, the capture antigen solution (50 μl, 0.5 μg/ml) was placed in a 96-well plate (Corning, Lowell, Massachusetts, USA) and incubated for 12 hr at room temperature. The wells were aspirated and filled with blocking buffer which contains 1% bovine serum albumin and 0.05% PBS-Tween 20, and incubated for 1 hr at room temperature. After the wells were washed 3 times with 0.05% PBS-Tween 20, human serum samples in blocking buffer at a dilution of 1:100 (vol/vol) were added to the wells. Four positive and 4 negative control serum samples were also added to each plate. After 2 hr of incubation at room temperature, the plates were washed 3 times with 0.05% PBS-Tween 20, and then peroxidase-conjugated anti-human IgG (Sigma; 1:2,000, vol/vol) diluted in blocking buffer was added. The plates were re-incubated for 1 hr at room temperature. The reaction was stopped by washing the plates as described above. To develop the color, 100 μl of 2,2’-azino-di-(3-ethyl-benzthiozoline-6-sulfonic acid) (ABTS) peroxidase substrate (Kirkegaard & Perry Laboratories; Gaithersburg, Maryland, USA) was added, and the plates were incubated for 30 min. The absorbance was measured at 405 nm, and the cut-off value for positivity was defined as the mean+ 2SD (standard deviation) of the negative control samples.
Calculation of the annual parasite incidence (API)
Malaria is classified as a Group III communicable disease that should be controlled by the Korean Government. Cases of malaria detected in private hospitals or clinics are reported to the local Public Health Center (PHC). The data collected by the PHCs are periodically provided to the Division of Infectious Disease Surveillance (DIDS) of the KCDC. Therefore, for this study, APIs of 2004 and 2005 were obtained from the DIDS. The API was calculated as the number of malaria-positive patients per 1,000 inhabitants for each of the study sites via microscopy. APIz=(no. of positive slides/total no. of slides) ×1,000.
Data analysis
Pearson’s correlation analysis was performed to examine the relationship between seropositivity and the API of P. vivax in a given year. The data were analyzed using SPSS software (version 17.0, SPSS Inc.; Chicago, Illinois, USA). A P-value of less than 0.05 was considered statistically significant. The correlation magnitude was interpreted as none (0.0-0.09), small (0.1-0.3), medium (0.3-0.5), or strong (0.5-1.0) [16].
RESULTS
The study locations are shown on the map in Fig. 1. All areas are near the DMZ, a known high-risk area for malaria. Blood samples were collected from participants residing in 23 villages in 3 cities (Gimpo, Paju, and Yeoncheon) located in Gyeonggi Province and from 6 villages in Cheorwon in Gangwon Province, South Korea. The total number of inhabitants in the study areas in 2004 was 92,246; thus, 1.92% of the whole population was sampled. In Paju, 1 malaria case was reported in 1993, and subsequently, 3,264 patients were reported up to 2013. In Gimpo, 1 patient was reported in 1995, and subsequently, 1,142 patients were reported up to 2013. In Yeoncheon, 2 patients were reported in 1995, and subsequently, 1,935 patients were reported up to 2013. In Cheorwon, 9 patients were reported in 1997, and subsequently, 1,388 patients were reported up to 2013. As shown in Fig. 2, the incidences of malaria patients reached its lowest levels in 2004, and then increased thereafter once a blood collection program had been initiated. The highest number of patients was reported in 2001 at 835 cases; however, only 90 cases were reported in the study areas (Fig. 2) (KCDC, personal communication).
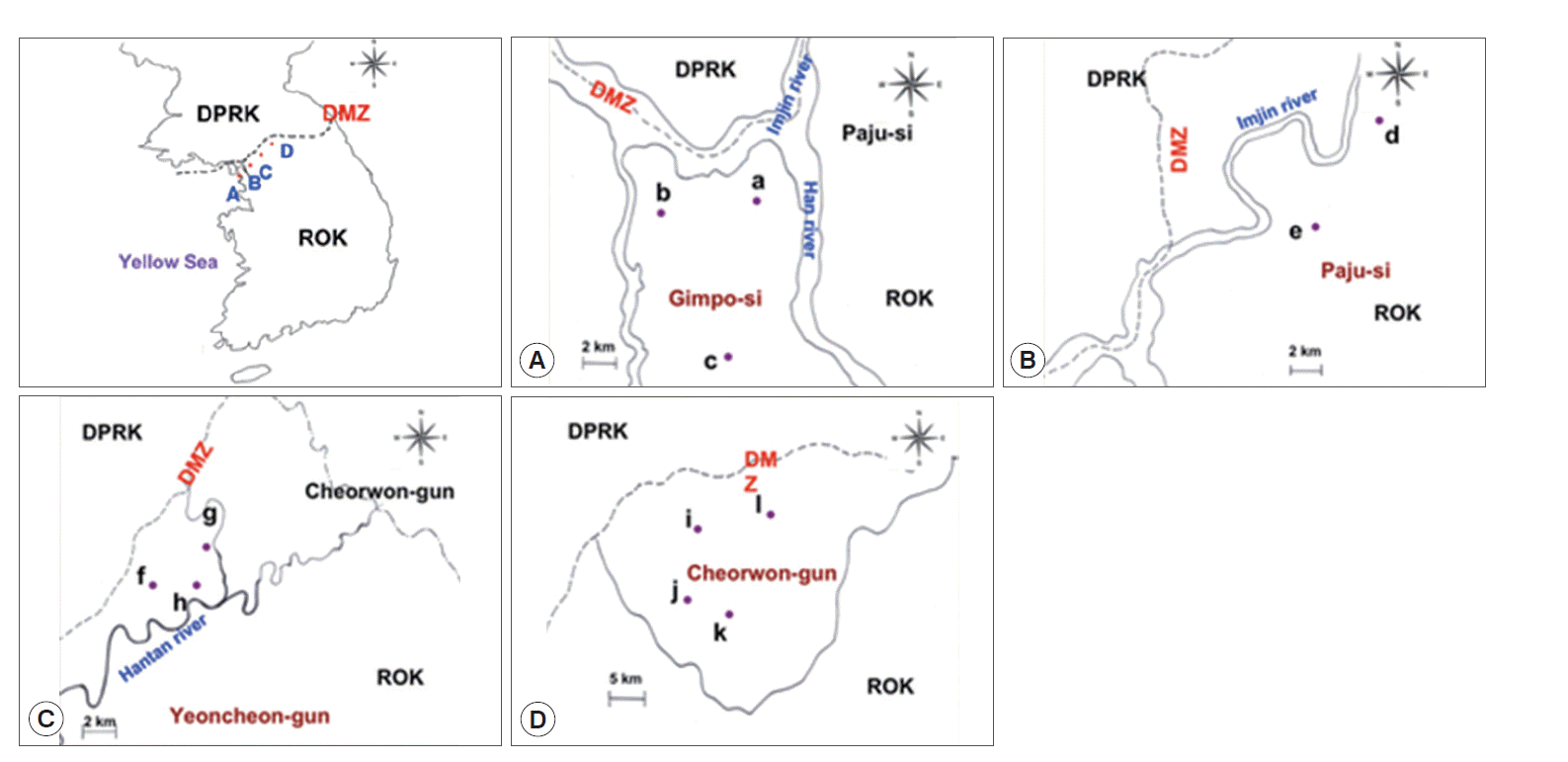
Study areas. (A) Gimpo-si, (B) Paju-si, (C) Yeoncheon-gun, and (D) Cheorwon-gun. a, Haseong-myon; b, Wolgot-meon; c, Yangchon-meon; d, Papyeong-myon; e, Munsan-eup; f, Baekhak-myon; g, Wangjing-meon; h, Misan-meon; i, Gimhwa-eup; j, Seo-myon; k, Cheorwon-eup; l, Geunnam-myon.
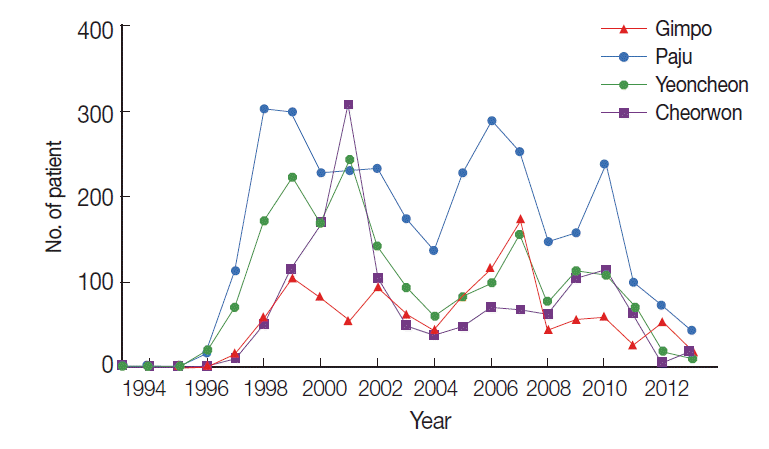
The number of malaria patients from 1993-2013 in the study areas. ●, Gimpo-si; ■, Paju-si; ▲, Yeoncheon-gun; ▼, Cheorwon-gun.
Of the 1,774 study subjects, 113 (6.4%) presented a positive CSP-ELISA response. Gimpo (Fig. 1A) displayed the highest positive CSP-ELISA rate (44/425, 10.4%), followed by Paju (Fig. 1B; 34/372, 9.1%), Yeoncheon (Fig. 1C; 19/451, 4.2%), and Cheorwon (Fig. 1D; 16/526, 3.0%). The API of 2004 was higher than that of 2005. The API was stable within each study area from 2004 to 2005. Yeoncheon presented the highest API in both years, followed by Cheorwon, Paju, and Gimpo (Table 1).
Of the 425 inhabitants of Gimpo, 44 (10.4%) presented a positive CSP-ELISA response. Haseong-myeon (Fig. 1A-a) presented the highest infection rate (7/53, 13.2%) based on CSP-ELISA, followed by Wolgot-myeon (Fig. 1A-b; 17/166, 10.2%) and Yangchon-myeon (Fig. 1A-c; 9.7%). Wolgot-myeon presented the highest API in 2004 (1.42) and 2005 (0.71). Haseong-myeon presented the second highest API in 2004 (0.69), but there were no malaria patients reported in 2005. Yangchon-myeon had the third highest API in 2004 (0.50), but the second highest in 2005 (0.25) (Table 2).
Of the 372 inhabitants of Paju, 34 (9.1%) presented a positive CSP-ELISA response. Papyeong-myeon (Fig. 1B-d) had a higher infection rate (25/140, 17.9%) than Munsan-eup (9/232, 3.9%) based on CSP-ELISA (Fig. 1B-e). Papyeong-myeon had a higher API than Munsan-eup in both 2004 and 2005. The correlations between the positive CSP-ELISA rates and API in 2004 (r=1.000, P=0.01) and 2005 (r=1.000, P=0.01) were significant (Table 3).
Of the 451 inhabitants of Yeoncheon, 19 (4.2%) presented a positive CSP-ELISA response. Baekhak-myeon (Fig. 1C-f) presented the highest positive rate (13/265, 4.9%), followed by Wangjing-myeon (Fig. 1C-g; 5/111, 4.5%) and Misan-myeon (Fig. 1C-h; 1/75, 1.3%), based on CSP-ELISA. Similarly, Baekhak-myeon had the highest API in 2004 (3.69) and then dropped to third place in 2005 (1.34). Misan-myeon had the second highest API in 2004 (2.79) but ranked first in 2005 (2.79). Wangjing-myeon had the lowest API in 2004 (1.60) and ranked second in 2005 (2.40) (Table 4).
Of the 526 inhabitants of Cheorwon, 16 (3.0%) had a positive CSP-ELISA response. Geunnam-myeon (Fig. 1D-l) had the highest infection rate (6/143, 4.2%), followed by Seo-myeon (Fig. 1D-j; 4/126, 3.2%), Cheorwon-eup (Fig. 1D-k; 4/142, 2.8%), and Gimhwa-eup (Fig. 1D-i; 2/115, 1.7%) based on CSP-ELISA. Seo-myeon had the highest API in 2004 (1.98), followed by Gimhwa-eup (1.54), Cheorwon-eup (0.86), and Geunnam-myeon (0.45). However, Gimhwa-eup had the highest API in 2005, followed by Geunnam-myeon (2.23), Seo-myeon (0.49), and Cheorwon-eup (0.35) (Table 5).
DISCUSSION
The survey areas of this study are regions affected by a reemerging malarial outbreak in South Korea: Gimpo, Paju, Yeoncheon, and Cheorwon, which are located within 10-15 km of the southern DMZ [8]. The DMZ is a 4-km-wide and 250-km-long corridor that extends across the middle part of the Korean peninsula. No civilians have been allowed to enter the DMZ for more than 50 years; therefore, natural landscape ecosystems and biodiversity are highly conserved in the DMZ [17]. The outbreak areas are expanding annually, both in the southern and eastern directions from the DMZ. The outbreak areas are believed to have originated from the northern part of the DMZ. Since human passage through the DMZ is almost impossible (although there are some exceptions in the Gaeseong Industrial Zone), the reemergence of malaria in this area is presumed to have originated not from the immigration of infected people from the north, but rather from mosquitoes infected with P. vivax that flew from the north. The corridor is heavily fortified on both sides of the buffer zones with land mines and barbed wire fences. Therefore, it is believed that these areas are exposed to vector mosquitoes. To estimate the prevalence of malaria exposure in these high-risk areas in Korea, the expression of 2 recombinant proteins were evaluated. One is CSP, which is a sporogony-stage protein that consists of the surface membrane common to all plasmodial sporozoites. CSP has a central immunodominant region consisting of short tandem-repeat amino acid sequences that contain multiple copies of the immunodominant B-cell epitope [9]. Because of its high immunogenicity and ability to induce a protective response in sporozoite-immunized experimental animals and humans, CSP is being investigated as a candidate molecule for the development of a human malaria vaccine. The immunodominant B-cell epitopes of CSP from a large number of isolates of P. falciparum of diverse geographical origin and of a smaller number of isolates of P. vivax have been examined and were found to be conserved within each species [18].
Interestingly, the maximum lifespan of the CSP antibody in human beings was determined to be 27 days in inhabitants of Thailand, and could not be boosted by additional exposure to CSP antigen, i.e., additional infection by anopheline mosquitoes [19]. These findings motivated us to consider the case of long incubation-period patients, who usually present with onset the year following infection after a 5-month-long winter season without mosquitoes. Furthermore, the mean incubation period of P. vivax has been reported to be as long as 279±41 days (range, 153-452 days) [20]. This means that these long incubation patients could display either the absence of or a reduced antibody level against CSP antigen. The percentages of cases with short or long incubation periods were 25% and 75%, respectively [8]. However, it is possible that the CSP antibody level was elevated in short incubation-period patients who presented with onset within a month after exposure to CSP antigen via infective anopheline mosquitoes. Therefore, the CSP antigen was selected among the many possible malaria antigens for this study. The hypothesis suggested by our malaria team is that the API in a given year accounts for both short incubation-period patients who were infected in the relevant year and long incubation-period patients who were infected in the previous year. Therefore, the positive anti-CSP antibody rates were compared with the APIs in the relevant and following years. In addition, sera obtained from inhabitants who lived in non-epidemic areas were assayed to determine the false-positive rate of CSP antigen by western blotting. Three of 28 samples displayed a positive reaction (10.7%) in preliminary experiments. However, the possibility that these 3 positive cases had never been in a malaria epidemic area in Korea could not be ruled out. Therefore, it was concluded that whether malaria patients had travelled in high-risk areas in Korea should be considered.
The incidence of malaria peaks in August after the rainy season and declines to baseline by mid-October. Therefore, blood collection was carried out between late-October and mid-December, when the active anopheline population has diminished. Antibody detection might provide useful information concerning P. vivax infection in a previously naïve population. Therefore, the correlations between the CSP-positive rate and incidence of malaria patients were compared in high-risk areas. The correlations between the positive CSP-ELISA rate and APIs of 2004 and 2005 were strongly negative, but the API of 2005 showed a much stronger negative correlation with CSP positivity compared to that of 2004 in all 4 cities (Table 1). The correlation between the positive rate of CSP-ELISA and API of 2004 was slightly negative, but was strongly negative with the API of 2005 in Gimpo-si (Table 2). The correlations between the positive rates of CSP-ELISA and APIs of 2004 and 2005 were strongly and significantly positive in Paju-si (Table 3). There was no correlation between the positive rate of CSP-ELISA and API of 2004 in Yeoncheon-gun, but a strongly negative correlation with the API of 2005 was observed (Table 4). The correlation between the positive rate of CSP-ELISA and API of 2004 was strongly negative in Cheorwon-gun, but there was no correlation with the API of 2005 (Table 5). The differences in these results might be related to the different malaria control programs in these areas. Malaria control is carried out by 4 different PHCs. Even with the main guidelines from the KCDC, each PHC has their own manual that takes into account local conditions of malaria prevalence and geographical characteristics. To evaluate malaria transmission in a given geographical region, many factors, such as temperature, mosquito density, vector capacity, climate, rainfall, and humidity, should be considered [21]. However, collecting such data requires great effort. Therefore, it is necessary to establish a cost-effective tool to analyze the current and future transmission patterns of malaria in specific areas using serodiagnostic methods. Parasitemia provides a classical means of measuring malaria endemicity, but patient incidence alone cannot provide a complete understanding of malaria prevalence since many factors affect malaria prevalence in Korea, such as the population density of mosquitoes, vectorial capacity, long to short incubation period ratio, symptomatic to asymptomatic patient ratio, differences in rainfall and temperature, and immunity of the community. The present study found that antibody detection methods were closely related to API values, which demonstrates the value of this method for understanding malaria transmission in a given area even without knowledge of the aforementioned factors.
In summary, antibody detection using CSP-ELISA may provide useful information regarding malaria prevalence in certain areas and individuals. These serological methods are useful for identifying areas that require malaria control and for evaluating the surveillance system in certain areas.
Acknowledgements
We are grateful to all blood donors and the staff members of the Public Health Centers in Gimpo, Paju, Yeoncheon, and Cheorwon, Korea. This work was supported by the Korean National Institute of Health and Inha University Research Fund (2014).
Notes
We have no conflict of interest related to this work.




