A Rare Human Case of Dirofilaria repens Infection in the Subcutaneous Posterior Thorax with Molecular Identification
Article information
Abstract
The emergence of Dirofilarial infections in Asia including Vietnam is a clinically significant threat to the community. We here report a rare case of subcutaneous Dirofilaria repens infection on the posterior thoracic wall in a young woman presenting a painful, itchy, and palpable nodule. The adult worm was identified by mitochondrial cox1 and nuclear ITS-2 sequence determination. The diagnosis was additionally confirmed by 16S rRNA sequencing of the endosymbiont Wolbachia pipientis commonly co-existing with D. repens. This is a rare case of subcutaneous human infection on the posterior thoracic region caused by D. repens.
INTRODUCTION
The genus Dirofilaria consists of at least 2 species of epidemiologically important human pathogens, Dirofilaria immitis (Leidy, 1856) and Dirofilaria (Nochtiella) repens (Railliet and Henry, 1911). These worms commonly cause Dirofilariasis in dogs, cats, and wild carnivores [1] and are globally recognized as etiological agents of zoonotic infections in humans, transmitted by vectors/mosquitoes (mosquito-borne zoonosis), including Anopheles, Culex, and Aedes [2,3]. D. immitis, that causes Dirofilariasis in dogs, cats, and humans, tends to distribute in temperate and tropical regions of the world, while D. repens infections are limited in Asia and Europe [1], including Vietnam [4,5]. Dirofilaria spp. can localize in almost any organs in carnivores and humans [1], including sucutaneous legions. However, to our knowledge, no previous report of human infection on the posterior thoracic region due to D. repens has been reported. Here, we report a rare case of subcutaneous posterior thoracic infection caused by D. repens diagnosed by morphology and molecular analysis.
CASE RECORD
On 14 March 2011, a 34-year old woman, visited a surgeon at a clinic in a suburban area of Hanoi, Vietnam with a painful swelling and itchy and palpable nodule on her posterior thoracic wall. She lived in an area where dogs and cats were outdoors allowed and several times exposed to mosquito bites. Three weeks before, on 26 February 2011, she suddenly noticed a swollen, tangible, subcutaneous nodule of the similar appearance as the current one at the same site but it has spontaneously disappeared days after. During the physical examination, a smooth, tangible, slightly movable, painful nodule of 1×2 cm in size was touchable under the skin on the posterior part of the thoracic wall, correlative with the vertebrae D11. The overlying skin was normal without any overt signs of inflammation and axillary lymph nodes were not enlarged. Routine laboratory test showed red blood cells of 3.9×106/mm3, white blood cells of 6.1×103/mm3 (the percentage of neutrophils, lymphocytes, and eosinophils were 60%, 20.7%, and 11.5%, respectively). No microfilaraemia was detected at this time in her blood samples by microscopy. By local excision under anesthesia, a well-circumscribed, encapsulated swelling nodule was revealed. After opening the capsule, a thin thread-like worm was noted and captured in 3 fragments which then were fixed in 10% formalin for morphological examinations and a piece was preserved in 70% ethanol for molecular identification.
A long, slender, whitish worm (Fig. 1A, B) excised from the nodule was approximately 10.5 cm in length and about 400 µm in its largest width, typical of the description of a female D. repens. Microscopy revealed a thick laminated cuticle with the characteristic longitudinal ridges and cross-striations (Fig. 1B). The anterior end was bluntly rounded and was of greater diameter than the posterior end (Fig. 1A). The body cavity contained a female reproductive system, with the bulbous vulva of about 1 mm from the anterior end, and ended in a uterine bifurcation. Following the surgical removal, symptoms were quickly resolved, and the patient was recovered without any consequences after a long-time followed up.
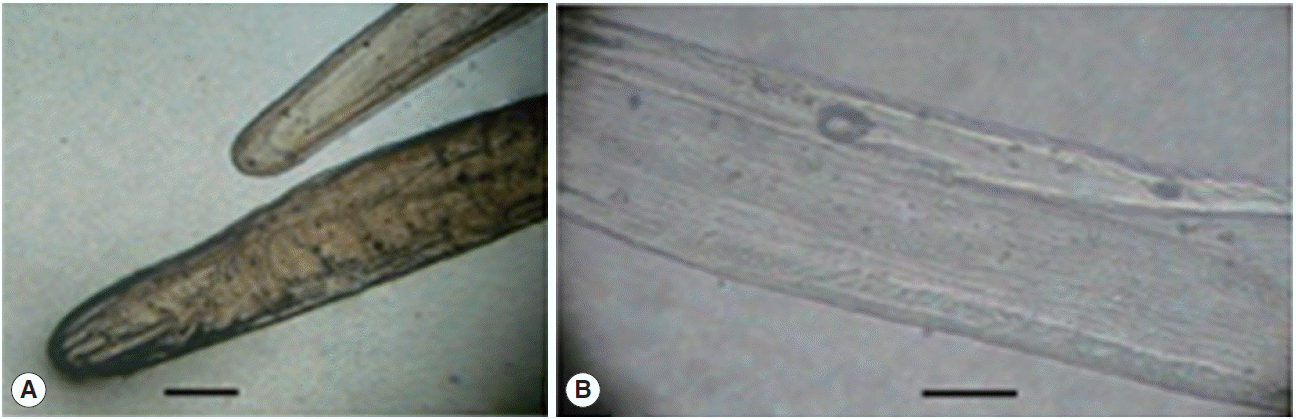
Photomicrographs of the Dirofilaria sp. sample collected from the posterior thoracic nodule of the patient, mounted in glycerin. (A) Anterior and posterior ends of the worm. (B) The outer surface of the worm's cuticle (×100). Scale bar=100 μm.
Total genomic DNA was extracted from an ethanol-fixed fragment of the excised worm by QIAamp DNA Mini kit (QIAGEN, Hilden, Germany). PCR of a portion of the mitochondrial cytochrome c oxidase I (cox1) gene was performed using the primer pairs (forward DIMREF, 5´ GATGTTGCCTTTGATATTGGG 3´; and reverse DIMRER, 5´ ACCAGCWGTWYACATATGATGACC 3´) designed based on the alignment of cox1 sequences of both Dirofilaria repens and D. immitis available in GenBank. The PCR, of 50 µl containing 25 µl PCR Master Mix from Promega, 2 µl of each primer (10 pmol/µl), 2.5 µl DMSO, 16.5 µl pure water, and 1 µl template (100 ng/µl), was carried out in a MJ thermal cycler PTC-100 (MJ Research, Watertown, Massachusetts, USA) with initiation at 94˚C for 4 min, then 35 cycles including denaturation at 94˚C for 30 sec, annealing at 55˚C for 30 sec, extension at 72˚C for 1 min; and a final cycle of 7 min at 72˚C to obtain a PCR product of 643 bp, and a 576 bp portion was used for alignment for taxonomic identification.
To support the identification, the presence of 16S rRNA sequence of Wolbachia sp. was obtained by PCR with the primer pairs designed hereby in this study, based on 16S ribosomal sequence of Wolbachia pipientis available in GenBank (WP16F: 5´-CCACACTGGAACTGAGATACG-3´ and WP16R: 5´-TGTAGCCACCATTGTAGCAC-3´).
DISCUSSION
The parasite recovered from the subcutaneous tissue of our patient was identified as D. repens on the basis of the morphology and molecular methods. The external longitudinal cuticular ridges differentiate D. repens from D. immitis, the other important human pathogens of the genus Dirofilaria [6]. However, identification of Dirofilaria spp. based only on the morphology is difficult; therefore, molecular methods supported to determine the species.
Molecular identification was carried out by using primers of cox1 and of nuclear internal transcribed spacer 2 (ITS2) [7], and additionally 16S rRNA identification of the endosymbiont W. pipientis commonly co-existing with Dirofilaria spp. [2]. Nucleotide cox1 sequence determination of the Vietnamese dirofilarial nematode revealed 96% identity to those of the Italian/Albanian D. repens strains (GenBank no. AM749230-AM749234; DQ358814; AJ271614; JF461458); and 89% to D. immitis (GenBank: AM749226; AM749227; AM749229; AJ537512; EU159111; EU163945; KC107805; AJ271613; DQ358815; FN391553) of different geographic origins. The cox1 amino acid sequence showed 99-100% similarity to all of the D. repens samples, while only 96% to D. immitis. High intraspecific variability was observed in D. repens cox1 sequences, while those of D. immitis species samples were identical (100% identity to each other), regardless of geographical distribution. Phylogenetic tree inferred from the 576 bp mitochondrial cox1 sequence of 20 strains including D. repens collected from the subcutaneous posterior thoracic nodule in this study, and 8 other D. repens, 10 D. immitis, and a nematode species O. lupi (as an out-group) indicated that the Vietnamese filarial sample clustered with cox1 sequences of D. repens, in a group, that was completely separated from the D. immitis and from O. lupi out-group sequences (Fig. 2). The genetic distances among the newly identified D. repens of Vietnamese patient and reference strains were calculated based on the Kimura 2-parameter model using MEGA5.2 software [8] (Table 1). The genetic distances among the Dirofilaria strains within each of D. repens or D. immitis, respectively, were very low, ranging from 0.000 to 0.039 for D. repens (Table 1, No. 1-9) and no variation (0.000) for D. immitis (Table 1, No. 10-19). There was relatively high taxonomic genetic distance, ranging from 0.103 to 0.117 between D. repens and D. immitis (Table 1, No. 1-9 vs No. 10-19); and 0.109 to 0.125 between Dirofilaria spp. and a nematode O. lupi, used for an out-group (Table 1, No. 20).
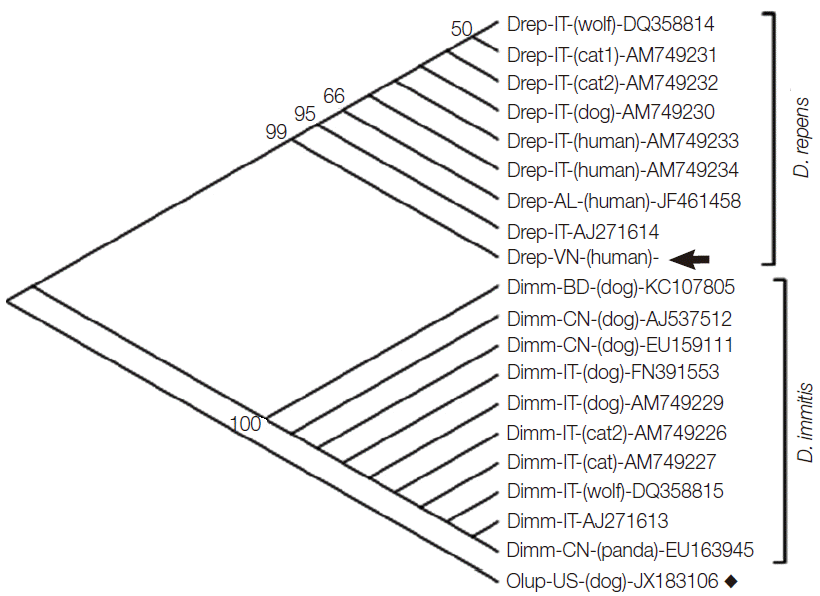
Phylogenetic tree inferred from the 576 bp-mitochondrial cox1 sequence of Dirofilaria repens collected from the subcutaneous posterior thoracic nodule of the patient, and 8 other D. repens, 10 D. immitis, and a nematode species O. lupi (as an out-group), constructed by MEGA5.2 software using the neighbor-joining method with bootstrap values of 1,000 replicates (values <50 were not shown) [3]. The 2 clusters of D. repens (Drep) and D. immitis (Dimm), respectively, are shown in separate groups, with the Vietnamese D. repens (indicated by an arrow); and an out-group species O. lupi (by square). The country origins of the samples are marked by codes of 2 letters (IT, Italy; VN, Vietnam; AL, Albania; BD, Bangladesh; CN, China; US, United States). Words in bracket indicate the host of the dirofilarial infections (where available), and the GenBank accession nos. are shown at the end of each sequence (for genetic distances, see data presented in Table 1).

Genetic distance of Dirofilaria repens and D. immitis strains (calculated by Kimura 2-parameter model)
By amplification with the primer pairs based on 16S ribosome, a sequence of 918 nucleotides was obtained and compared with those of the previously known W. pipientis 16S rRNA sequences, i.e., one amplified from a D. repens (Italian strain, GenBank: AJ276500) and several from D. immitis strains (Z49261, AF088187, and AF487892), available in GenBank. The comparative sequence analysis revealed 96-97% identity among the endosymbionts and accurately confirmed the presence of the endosymbiont W. pipientis in the Vietnamese D. repens.
The species identification of this Vietnamese D. repens, thus, was completely accurate using both the mitochondrial and nuclear nucleotide analysis and distance-based/phylogenetic approaches. To date, of 3,192 human dirofilariasis cases reported worldwide, 372 pulmonary and 1,410 subcutaneous/ocular infections were documented; and due to aberrant migration of the microfilariae, Dirofilaria spp. can localize in almost any organs in carnivores and humans [1]. Lesions caused by D. immitis are focused mainly in the heart (dogs and cats) and in the lungs and skin (in humans, presented as pulmonary and subcutaneous forms), whilst D. repens tends to localize in multiple organs, mainly in the subconjunctiva [5,9,10], lungs and coronary artery [11-13], soft tissues as the breast, penis, spermatic cord, epididymis, and scrotum [14-17], brain, liver, lymph nodes, muscles, and anterolaterial neck [5,18-21], and rare places, i.e., causing intravitreous infection in humans [22]. To our knowledge, this is the first case of human infection on subcutaneous posterior thoracic region due to D. repens reported.
In humans, normally a single subcutaneous nodule and lesions have been developed during weeks and described as little granulomatous inflammatory lesions [5]. In the case reported here, the time and the means of transmission by which the patient caught D. repens infection is unclear, probably by mosquitoes bites (infected by larvae from the outdoor dogs/cats wandering in the suburb). Unfortunately, no surveys of microfilariae in dogs/cats have been conducted yet. To date, 12 Dirofilaria infections of the conjunctiva (eye subcutaneous and subconjuctiva) have been detected in Vietnam [4,5]. The case of posterior thoracic localization hereby presented is a rare record and all of the Vietnamese infections documented are autochthonous, caused by solitary D. repens.
In our case, molecular classification of the causal agent confirmed it to be D. repens using complex approaches, including morphological, historic, mitochondrial cox1, nuclear ITS-2, distance-based/phylogenetic analysis, and supported by the endobiosymbiont, W. pipientis, 16S-rRNA based identification. Regarding high risk of human dirofilariasis (imported Dirofilaria is not exclusive) in Vietnam, detailed national surveys involving pathological, epidemiological and etiological information on Dirofilaria species, animal/host reservoirs, vectors/mosquitoes, provincial distribution and improvement of routine laboratory diagnostic approaches, including molecular identification/discrimination of zoonotic Dirofilaria are needed for early control of the emerging dirofilarial infections.
Acknowledgements
Thanks are expressed to colleagues at Immunology Department, Institute of Biotechnology, Vietnam for their laboratory technical assistance. This work was partially supported by the National Foundation for Science and Technology Development (NAFOSTED) (grant no. 106.06-2012.05, to Le TH).
Notes
We have no conflict of interest related to this work.