Diagnosis, Treatment and Clinical Features of Cutaneous Leishmaniasis in Saudi Arabia
Article information
Abstract
Cutaneous leishmaniasis (CL) has been one of the most common parasitic diseases in Saudi Arabia. This study exhibits the clinical features, diagnosis, cytokine profile and treatment of CL patients in Al-Taif province. Ninety CL suspects at a tertiary care general hospital were enrolled in one-year study. Patients were interviewed, clinically-examined, and subjected to laboratory tests: skin scraping smear microscopy, OligoC-TesT commercial PCR (Coris BioConcept) and kinetoplast DNA (kDNA) PCR for Leishmania diagnosis. Interferon-gamma (RayBio; Human IFN-γ) and nitric oxide (NO) levels in patients’ sera were evaluated before treatment with sodium stibogluconate (pentostam) with 20-day intramuscular drug regimen. Positive rates of microscopy, commercial PCR and kDNA PCR were 74.4%, 95.5% and 100%, respectively. Patients came to hospital mostly in winter (45.0%). CL was frequently exhibited in Saudi patients (78.8%), male gender (70.7%), age <20 years (50.0%), rural-dwellers (75.5%) and patients with travel history (86.6%). Lesion was mostly single ulcer (93.3%), occurred in the face (67.7%). Upon pentostam treatment, 85.1% of ulcers showed rapid healing signs. Levels of IFN-γ and NO were significantly higher in the healing than the non-healing cases (P<0.001). The kDNA PCR proved more sensitive than microscopy and OligoC-TesT commercial PCR. Our results open perspectives for IFN-γ use as a biomarker predicting treatment response.
INTRODUCTION
Cutaneous leishmaniasis (CL) is a vector-borne protozoan infection affecting a large number of people in several countries [1]. Around 1.5 million CL new cases are emerging annually and approximately 350 million people are at risk [2]. The great number of CL cases occurs in Algeria, Brazil, Afghanistan, Iran, Peru, Syria and Saudi Arabia. The disease is often asymptomatic, but it can exhibit symptoms very similar to that of many other skin diseases [3].
The progress of CL and its clinical outcomes are strongly-influenced by the host immune response mounted towards the causative parasite [4]. Interferon gamma (IFN-γ) is a major cytokine involved in macrophage activation. Upon activation, the macrophages produce nitric oxide (NO), a potent mediator involved in intracellular and extracellular Leishmania killing [5].
Diagnosis of CL is usually done through demonstration of the leishmanial parasite in skin smears or biopsy with direct microscopy-based detection methods that commonly lack sensitivity and specificity [6]. Several PCR-based assays that allow both parasite detection and species identification, with a high degree of sensitivity and specificity, have been developed [7].
CL skin lesions are often self-healing, but sometimes mandate treatment. The pentavalent antimonial medications remain the standard treatment in many parts in the world [8]. Nonetheless, these compounds are frequently blamed for their serious side-effects and the reported possibility of treatment-failure, resistance and skin lesions relapse [9].
In this study, we presented a comprehensive report describing the clinical features, diagnosis, cytokine profile and the antimonial treatment’s response of CL using cases with suspected leishmanial lesions from Al-Taif province, western Saudi Arabia.
MATERIALS AND METHODS
Setting and population
This descriptive study was carried out in Al-Taif region, an area of 13,840 km2, situated west Saudi Arabia (Fig. 1). The climate in this high altitude region is variable and the rain usually falls in late summer and autumn seasons. This study was conducted on 90 patients with active skin lesion(s) suspicious for CL. Cases were selected from those patients presented to the Dermatology department at King Feisal, a referral tertiary level general hospital in Taif during the period between September 2016 and August 2017. CL patients with history of prior therapy to the skin lesion(s), who had co-morbid diseases like cancer, chronic renal or liver diseases or infections as viral hepatitis or human immunodeficiency virus, was excluded from participation in the study.
Ethical considerations
An approval of Institutional Review Board (IRB) at Al-Taif University and King Feisal General Hospital (RAC #207004) was declared before the initiation of the study. In addition, a written informed consent was obtained from each participant after a detailed explanation of the study’s purpose, procedures, potential risks and benefits.
Clinical examination
Patients were interviewed with structured questionnaire and clinically-examined by a specialized dermatologist. The questionnaire comprised, in its first part, information about the patient’s socio-demographic features (age, gender, residence, nationality, animals in or near the house and leishmaniasis-endemic areas visited 1–3 months before eruption of the skin lesions) and in its second part, information about the patient’s medical history (prior medications, home remedies, concomitant diseases and lesion’s evolution time). The lesion’s evolution time was calculated by the time interval from the lesion’s eruption day till the patient’s consultation day.
The clinical examination involved tissue-affected, lesion characteristics (number, size, site and appearance). The lesion’s size was measured through 2 crossing diameters by a metric caliper. For patients with multiple lesions, the surface area was reported as a mean size of the total lesions.
Microscopic examination for Leishmania
Scraping materials were taken from the patient’s lesion and divided into 2 parts: one part was directly used for microscopic examination and another part was stored at 4°C for subsequent molecular parasitological diagnosis. Tissues were scraped from the active edge of the lesion by using a sterile lancet. The scraped material was smeared on a slide glass, fixed with one drop of methanol, stained with Giemsa stain and examined under a light microscope for leishmanial amastigotes, as previously described [10].
Molecular detection of Leishmania
Nnucleic acid of the stored scraping materials were extracted and purified using a genomic DNA purification Kit (Ferments, UK) according to manufacturer’s protocol. DNA was eluted with 50 μl elution buffer and kept at −20°C until PCR analysis. The first PCR analysis was done using Leishmania OligoC-TesT commercial kit (Coris Bioconcept, Gembloux, Belgium). The second PCR was kinetoplast DNA (kDNA) PCR, an in-house semi-nested conventional PCR. Amplification reaction and products analysis were performed with reference to a previous protocol [11].
Assay on IFN-γ and NO in sera
All participants were asked to give blood sample for assays on IFN-γ and NO levels in their sera before treatment. After overnight fasting, 5 ml of venous blood were withdrawn, kept at room temperature for 30 min. Serum was transferred to sterile tubes, centrifuged at 3,000 rpm for 10 min at 4°C and kept at −20°C. Level of IFN-γ was determined using RayBio® Human ELISA commercial kit (Norcross, Georgia, USA) following the manufacturer’s protocol.
Serum nitrate concentration, as a stable end-product of nitric oxide, was measured by an endpoint one-step enzymatic assay using nitrate reductase. The concomitant reduction of nitrate to nitrite by NADPH was monitored by the oxidation of the coenzyme and the decrease in absorbance at 340 nm (μmol/L).
Patients treatment and follow-up
Patients eligible for treatment were selected and sodium stibogluconate (Pentostam, GlaxoSmithKline, Uxbridge, UK) was administered intramuscularly at a dose of 20 mg/kg/day for 20 days. Ulcerative lesions were observed for healing during the intramuscular injections. Resolution signs of the lesions included inflammatory signs (skin erythema, edema and hardening) and size regression (partial or complete scarring, re-epithelialization).
Statistical analysis
Collected data were coded, tabulated and statistically analyzed using SPSS 19 program (SPSS, Chicago, Illinois, USA). Descriptive data were analyzed as frequency, percentage, and mean±standard deviation (SD). Serum level of IFN-γ was expressed as non-parametric variable and compared between groups using Mann-Whitney to test significant difference at P <0.05.
RESULTS
Socio-epidemiological features
Ninety CL suspects were identified and investigated in our study, with an estimated average annual frequency rate of 7.5 cases per 100,000 inhabitants. Cases registered all the year around, majority of cases were recorded in winter (45.0%), reached a peak at February/March (16.6%) and March/April (13.3%) interval periods. The cases decreased in other months and minimum in August (3.3%) and November (4.4%) (Fig. 2).
Of all CL patients, investigated, 77.7% were male (mean age=28.40±21.08) and 22% female (mean age 42.5±21.28). Patients were recruited into 4 age groups and their demographic characteristics were described in relation to their age groups (Table 1). Majority of cases were below 20 years (50.0%), coming from remote rural areas (75.5%) and had a travel history to leishmania-endemic areas inside or outside the country (86.6%). Significant difference was not observed in these 3 variables between the patients’ age groups (P >0.05, chi-square).
Infection appeared more frequently in Saudi people (71; 78.8%) than in non-Saudi (21.1%), with high significant differences observed among age groups and patient’s nationality (P <0.001). Moreover, 68.8% cases reported contact with domestic animals like dogs, sheep, goats, cattle, buffaloes, donkey or chickens in or nearby their houses, significant difference observed between different age groups (P <0.05).
Clinical characteristics
Considering the leishmanial lesions, examined, all were confined to the patients’ skin with no mucosal tissue involvement noticed. The mean number of lesions was 1.08±0.356 (mean±SD) and the mean surface area of 6.35±3.01 cm2. The lesion’s evolution time ranged between 3 and 15 months (mean±SD=5.96±2.53). The majority of lesions were of 3–6 months durations (85.5%). Most of the patients (84; 93.3%) presented with single lesion, while 6 patients developed 2–3 lesions. The highest proportion of lesions (85; 94.4%) was of ulcerative type while 5.5% of lesions were nodular. Lesions affected exposed body parts, mainly the face (61; 67.7%), followed by the upper limbs (15; 16.6%) and the lower limbs (14; 15.5%). Table 2 shows the clinical features of the leishmanial skin lesions relative the different age groups. The mean surface areas of lesions were below 5 cm in 35 (38.8%) of cases, 5–10 cm in 54 (60%) patients and more than 10 cm in just one case. The ulcerative lesions looked dry with scales in 85.8% (73/85) and wet in 14.1% (12/85) of cases. The lesions were exclusively-found on exposed body areas: the face in 67.7%, the lower limbs in 15.5%) and the upper limbs in 16.6% of patients. The facial lesions were found frequent in young patients below 19 years (38/61; 62.2%), while the limbs lesions were described more in older ages (≈ 86%), with highly significant differences observed among the patients age groups (P =0 .000273, chi-square).
Diagnostic tests results
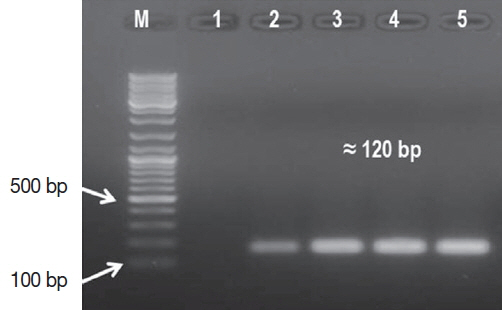
The Leishmania amastigote forms were exhibited in 67 (74.4%) cases by Giemsa stained smear microscopy. In comparison, the leishmanial DNA was identified in 86 (95.5%) cases with the OligoC-TesT. The assay picked 19 CL cases more than microscopy. The semi-nested kDNA PCR assay identified the remaining 4 CL cases, achieving 100% sensitivity (Fig. 3). Importantly, all leishmanial cases identified by microscopy were also positives for the parasite DNA.
Treatment and follow up results
Forty-seven (52.2%) patients were found suitable for treatment with sodium stibogluconate. All patients were followed-up at the end of therapy. Forty patients (85.1%) displayed clinical signs for partial or complete resolution of their skin lesions. For IFN-γ and NO serum levels analysis, these patients were allocated as group (A). The remaining patients (n=7) had no resolution signs for their skin lesions and recruited for the same purpose as group (B) for the above purpose.
IFN-γ and NO levels
Before pentostam therapy, the levels of IFN-γ and NO in patients’ sera were higher in group (A) than in group (B). Nonparametric Mann-Whitney test was used for comparative studies (Table 3). Statistically, the differences observed between the 2 groups were highly significant (P =0.000, 2-tailed).
DISCUSSION
During this relatively-short study period, 90 patients with active leishmanial skin lesions were identified in the study’s setting. At first glance, the absolute number of cases detected per one year seems relatively large, inconsistent with previous reports from the same setting [12]. Factors like the increasing urbanization close to the nearby endemic foci, the growing population’s size and the frequent population’s movement could be explanations. Moreover, the progress made in disease’s diagnosis and the increase in population’s awareness towards the value of early treatment may be additional explanations. Taken together, it was clear that CL in the study setting is more frequent than perceived in the literature and mandate further attention from the health authorities.
CL cases were reported all the year around, but most of the cases were reported in the winter, in agreement with previous Saudi studies [13,14]. Conversely, in a study, carried out in southeastern Tunisia, most of the cases were recorded in the summer [15]. The local climate and its effects on the host-vector activities could explain seasonality variations among different studies [16]. All CL lesions were confined to the skin with no mucosal tissue involvement or nodular dissemination noticed in all cases, consistent with previous studies [17,18] and inconsistent with others [19,20].
The patient’s young age, male gender, rural residence, and frequent travel were proven risk factors for CL in the study’s setting. Although no age was found immune to infection, patients below 20 years were the most susceptible age group. Identical observation has been displayed in an earlier report [21]. In contrast to our finding, patients aged 20–30 years have been described as a high - risk group, in one study [22] while older patients (40–60 years) were the most vulnerable group in another [18]. Regarding gender distribution, a definite male preponderance, with 3.5: 1 male to female ratio, was defined in our study. The same path has been widely perceived in other Saudi [13,14] and non-Saudi endemic loci [23]. Inconsistent with our finding, an equal distribution of CL cases between males and females has been also reported in one study [24] and the female gender predominance has been reported in another [25]. Perhaps, the above striking differences among studies are related to the cultural difference, behavioral patterns and occupational activities of the studied populations.
Also in our study, the microscopic examination of stained smear prepared from lesion’s scraping displayed sensitivity of 74%, higher than that previously reported. Sensitivities of 42%–70% have been reported in earlier studies [23,25]. All skin scraping samples that diagnosed as negatives by microscopy were proved positives for leishmanial DNA by one of the 2 PCR assays. The kDNA PCR was more sensitive than the OligoC- TesT, consistent with one study [26]. The high sensitivity of the kDNA PCR could be due to the high copy number of the selected gene, the highly conserved nature of the targeted DNA sequence targeted and the adopted semi-nested PCR format.
Furthermore, pentostam® with the 20 day-intramuscular therapeutic regimen was effective in treating 85% of cases. Lower cure rates have been reported in endemic regions elsewhere in the country [27,28]. Factors like the therapeutic regimen, the causative leishmania species, the lesion’s severity and duration, and the patient’s immune and health status, all can explain the variations of the drug efficacy displayed among studies [29].
Before pentostam® treatment, highly significant levels of IFN-γ and NO were exhibited in healing than non-healing patients groups, in agreement with an earlier study [30]. Conversely, Taheri et al. [31] have reported statistically non-significant difference regarding serum IFN-γ between CL cases and a group of healthy individual enrolled in the study as controls. Despite this discrepancy, our finding opens 2 perspectives for IFN-γ use as a biomarker capable to diagnose cases and predict responders from non-responders, and as an adjuvant medication to pentostam for treating resistant cases.
In fact, this study was not without limitations. Our results could be subjected to selection bias as the study’s population was hospital-based, or exposed to recall bias, because some findings relied solely on the patients’ memories. Due to cultural habits, the number of male and male participant were not adequately matched, thus, the sex distribution of CL could be also biased. Lastly, because of the study’s short period, post-treatment follows up visits could not be delivered in a way that allows us to comment on treatment resistant and relapsed cases.
To conclude, CL is prevalent in Taif region and the registered number of cases is rising all the year around, especially in the winter. All CL cases, evaluated, are of a localized skin lesion with no mucosal tissue involvement or papulo-nodular dissemination. Young age, male gender, rural residence, travel to endemic foci, all were determined as risk factors. Most of cases presented with a single ulcerative lesion in the face, followed by the upper and lower limbs. Moreover, the kDNA PCR assay proved most sensitive diagnostic tool, followed by the OligoCTesT kit and microscopy. Pentostam® with the adopted regimen proved effective in treating 85% of cases, especially those IFN-γ and NO levels in their sera before treatment initiation. We recommend more in-depth field and longitudinal studies in the near future to support our study’s findings.
ACKNOWLEDGMENTS
This research was supported by a grant (Project No. 1-437-4895) through the Research Support of Taif University, Saudi Arabia. The authors acknowledge the valuable assistance provided by Dr Ahmed M. Khalifa, for his help and support while doing the study’s statistics.
Notes
CONFLICT OF INTEREST
All authors declare no conflict of interest.