ELISA detection of IgG antibody against a recombinant major surface antigen (Nc-p43) fragment of Neospora caninum in bovine sera
Article information
Abstract
An ELISA was established to measure bovine IgG directed against the recombinant antigenic determinant of Nc-p43, a major surface antigen of Neospora caninum. In a previous study, two thirds of the C-terminal of the molecule was expressed as a 6 × His tagged protein (Ncp43P) for ELISA using 2/3 of the N-terminal of SAG1 from Toxoplasma gondii as a control (TgSAG1A). Among 852 cattle sera collected from stock farms scattered nation-wide, 103 sera (12.1%) were found to react with Ncp43P positively, but no positive reaction was observed with TgSAG1A. This study shows that Ncp43P could be available as an efficient antigen for the diagnosis of neosporosis in cattle. Furthermore, it together with TgSAG1A, could be useful for the differential diagnosis of N. caninum and T. gondii infections in other mammals.
Neospora caninum is an apicomplexan protozoon and an important cause of abortion in cattle worldwide (Dubey, 2003). Diagnosis is based on histopathologic and immunohistochemical evidences on the aborted fetus, but in many cases when fetal tissues are unavailable, it should be based on serology. Serological diagnosis has advanced considerably from the early development of IFAT (Dubey et al., 1988) and ELISA (Pare et al., 1995; Williams et al., 1997).
Recently, we expressed the Nc-p43 gene from a Korean strain of N. caninum (KBA-2) in bacteria (Kim et al., 2000), and demonstrated that the antigenic domain of Nc-p43 is localized in the C-terminal 2/3 parts of the molecule (Son et al., 2001). When the purified GST fused C-terminal 2/3 parts (P fragment) of Nc-p43 was used as an antigen in ELISA, a high level of background absorbance was detected, possibly due to the non-specific binding of antibodies to GST. Therefore, we undertook to substitute GST with 6 × His, a relatively shorter and less characteristic tag, to express an antigen for use in ELISA. In addition, the antigenic N-terminal 2/3 fragment of SAG1 from Toxoplasma gondii (Nam et al., 1996) was added to the plate as a control in an attempt to differentiate T. gondii, a neighboring apicomplexan infections.
PCR was used to amplify the DNA fragments of the C-terminal 2/3 fragment of Nc-p43 from N. caninum using 5'-GTA GGT ACC AAA GAG TGG GTG ACT GGA-3' as forward primer and 5'-GGT AAG CTT TGC ATC TCC TCT TAA CAC-3' as reverse primer, which amplified a 732 bp fragments. For the N-terminal 2/3 fragment of SAG1 from T. gondii, 5'-GTT GGT ACC GAT CCC CCT CTT GTT GCC-3' was used as forward primer and 5'-GGT AAG CTT GAC TCC ATC TTT CCC GCA-3' as reverse primer to amplify a 516 bp DNA fragments (Fig. 1). Both DNA fragments were treated with Kpn I and Hind III and inserted into pQE30 vector (Qiagen, Valencia, CA, USA). The plasmids were then used to transform the M15 strain (Qiagen) of Escherichia coli. The C-terminal 2/3 fragment of Nc-p43 was expressed as a 26 kDa protein (Ncp43P) on IPTG induction and the N-terminal 2/3 fragment of SAG1 as a 19 kDa protein (TgSAG1A) as shown in Fig. 2.
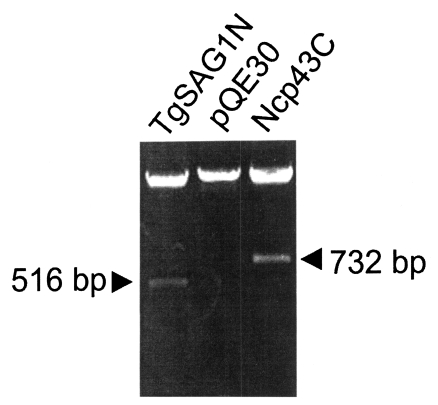
Cloning of genes from the C-terminal 2/3 fragment of Nc-p43 from Neospora caninum (Ncp43C) and from the N-terminal 2/3 fragment of SAG1 from Toxoplasma gondii (TgSAG1N) in pQE30 vector.
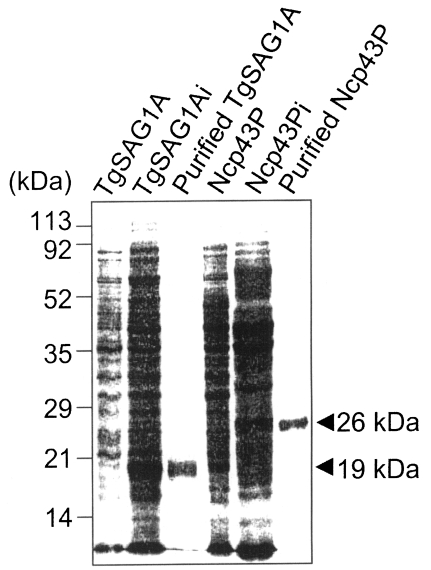
SDS-PAGE of M15 strain transformed with Ncp43C (Ncp43P) and TgSAG1N (TgSAG1A) plasmids, which were expressed by IPTG induction (i). Ni-NTA column purified antigens were represented by 26 kDa and 19 kDa bands. Numerals on the left indicated molecular mass as kDa.
Recombinant proteins were purified by passing through a Ni-NTA column (Qiagen) and used in ELISA at 5 µg/ml. ELISA was performed according to the method of Bae et al. (2000). Bovine sera were diluted 1:100 in PBS/Tween-20. Heat inactivated normal rabbit serum (10%) in PBS/Tween-20 was used as a blocking buffer and HRP-conjugated goat anti-bovine IgG antibody (Sigma Chem Co., St. Louis, MO, USA) was diluted to 1:10,000. The cut-off value for positive reactions was calculated as 0.32 for Ncp43P and as 0.30 for TgSAG1A based on assays of sera selected as negative by ELISA and western blot (Bae et al., 2000).
A total of 852 cattle sera were collected from stock farms randomly selected in 9 administrative provinces in 2001. Cattle sex and age were not identified. Of the sera samples, 103 (12.1%) sera reacted with Ncp43P positively, but not TgSAG1A (Table 1). Differences were observed in the prevalence rate of N. caninum infection among the studied provinces but these were not statistically significant. In particular, sera of cattle from Jeju-do were free of N. caninum infection.
Ncp43P and TgSAG1A could be useful in combination for the diagnosis of neosporosis in cattle. Furthermore, these two antigens could be available for the differential diagnosis between N. caninum and T. gondii infection in mammals.
Notes
This work was financially supported by the Ministry of Agriculture and Forestry (399002-3) of the Republic of Korea.
