Austropeplea ollula (Pulmonata: Lymnaeidae): A new molluscan intermediate host of a human intestinal fluke, Echinostoma cinetorchis (Trematoda: Echinostomatidae) in Korea
Article information
Abstract
Three freshwater snail species of the family Lymnaeidae have been reported from Korea, Radix auricularia coreana, Austropeplea ollula and Fossaria truncatula. Out of 3 lymnaeid snail species, A. ollula was naturally infected with the Echinostoma cinetorchis cercariae (infection rate = 0.7%). In the experiments with the laboratory-bred snails, F. truncatula as well as A. ollula was also susceptible to the E. cinetorchis miracidia with infection rates of 25% and 40%, respectively. All of three lymnaeid snail species exposed to the E. cinetorchis cercariae were infected with the E. cinetorchis metacercariae. It is evident that A. ollula acts as the first molluscan intermediate host of E. cinetorchis in Korea, and F. truncatula may be a possible candidate for the first intermediate host of this intestinal fluke. Also, three lymnaeid snail species targeted were experimentally infected with E. cinetorchis metacercariae.
INTRODUCTION
Life cycle studies of E. cinetorchis Ando and Ozaki, 1923 (Trematoda: Echinostomatidae), first described as a new species by Ando and Ozaki (1923), were carried out in Korea mostly after Seo and his colleagues (1980) reported a human case of echinostomiasis cinetorchis (Miki, 1923; Seo et al., 1980, 1984; Lee SH et al., 1988; Lee et al., 1990). This human intestinal fluke is morphologically characterized by a head crown with 37-38 collar spines and especially with 6 spines on the ventral lobe (Ando and Ozaki, 1923; Seo et al., 1964; Lee et al., 1992). A total of 6 human cases of infection with this trematode have been reported in Korea (Seo et al., 1980; Ryang et al., 1986; Lee SK et al., 1988; Ryang, 1990; Son et al., 1994).
Three freshwater lymnaeid snail species, such as R. auricularia coreana, A. ollula, and F. truncatula, belonging to the family Lymnaeidae have been reported so far in Korea. However, only a planorbid snail, Hippeutis cantori was listed to be naturally infected with the cercariae of E. cinetorchis (Ahn et al., 1989), and was also examined experimentally as the first and second intermediate hosts of this trematode (Lee et al., 1990). As the second intermediate hosts of this trematode, one of the loaches named Misgurnus anguillicaudatus (Koga, 1938) and several freshwater snail species such as H. cantori, Radix auricularia coreana, Physa acuta and Cipangopaludina chinensis malleata were listed by the Korean parasitologists (Lee SH et al., 1988; Ahn et al., 1989; Chung and Jung, 1999). Recently, Segmentina hemisphaerula was confirmed naturally and experimentally as the first and second intermediate hosts of this trematode (Chung et al., 2001).
The present study was carried out to observe the natural infection of the cercariae of E. cinetorchis from the lymnaeid snails collected from the local sites, and to test the susceptibility of laboratory-bred Korean lymnaeid snails to infection with the miracidia and cercariae of E. cinetorchis.
MATERIALS AND METHODS
Cultivation of the snails
Several local collections of three lymnaeid snail species were mainly used in the culture studies. The snails were maintained mainly in conventional aquaria. Initially, tap water was conditioned before use simply by adding 4-5 drops of 5% sodium thiosulfate solution to each aquarium for dechlorination (pH, 6.5-7.0). This water was aerated for one week prior to use. The aquaria were placed on shelves in a shelving unit and exposed alternately to 12 hr of artificial light provided by a 15-watt cool, white fluorescent tube and 12 hr of darkness. An automatic timer controlled the light cycle. The water of all the glass aquaria or the plastic trays for the juvenile snails was continuously aerated by bubbling air from a air compressor through the water. The water temperature for the aquaria ranged from 25-27℃. Lettuce and Tetra SML flakes (Tetra SML®, Tetra Co., D-452 Melle, West Germany) were supplied to the lymnaeid snails twice a week (Chung, 1984).
Observation of naturally emerging cercariae from snails
Three species of lymnaeid snails collected from various local areas were first examined for the emergence of trematode cercariae. For the observation of trematode cercariae from snails, each snail was placed in a well of 24-well plastic plate (Emerald Biostructures Inc., Bainbridge Island, WA, USA) containing the appropriate amount of aerated tap water, kept under a fluorescent light for 2 hr and examined for the presence of shedding cercariae. Later, the snails were crushed for further examination of cercariae inside the snails.
Cercarial infection in the second intermediate host
Three species of laboratory-bred lymnaeid snails infected with cercariae, were examined in order to obtain metacercariae of E. cinetorchis. A total of 50 cercariae shed from lymnaeid snails were exposed to each experimental snail of S. hemisphaerula, known as the second intermediate host (Chung et al., 2001) and the infected specimens were examined for the detection of metacercariae. After cracking the snail shell and removing the pieces of shell, the animal bodies were dissected and digested in artificial gastric juice for 1 hr at 37℃. The metacercariae were collected from snails every week from two weeks after the cercarial challenge. The number of Echinostoma metacercariae per known volume was counted under the low power (×100) of a light microscope.
Metacercarial infection in the final host
A total of 50 metacercariae was fed orally using a tuberculin syringe connected with a plastic tube to each rat (Sprague-Dawley strain, 120 g of body weight). Rat feces was examined daily for eggs of E. cinetorchis. Immediately after finding the eggs, the rats were killed by cervical dislocation and dissected. The small intestines of the rats were removed. The worms were collected under the dissecting microscope and their numbers were determined. Worms were fixed with 10% formalin under the cover slip pressure and stained with Semichon's acetocarmine. The stained worms were observed for morphological comparisons with the standard parasitic specimens.
Miracidial and cercarial infection in the laboratory-bred snails
Parasite-free snails were reared in the laboratory to obtain their laboratory-bred offsprings. The snail eggs of each species were collected and cultured to get laboratory-bred adult snails in a batch which were employed for the susceptibility experiments to the target parasite E. cinetorchis. The infected rats were used as the source of eggs for the susceptibility studies of snails. For the susceptibility experiments of E. cinetorchis miracidia to the laboratory-bred snails, eggs (Fig. 1B) were collected by tearing the worms. The eggs were rinsed 3 times with distilled water and transferred to the petri dishes containing conditioned water with a few drops of Fungizon solution (Gibco Life Technologies Inc., Grand Island, New York). The eggs were incubated in the conditioned water at 26℃ in a dark incubator with aeration. Some hatched miracidia were used to challenge the three lymnaeid snail species targeted in this study. Each snail was exposed to a dose of twenty miracidia hatched from the eggs of E. cinetorchis. Twenty days after miracidial infection, the release of cercariae was examined from the snails kept under the fluorescent illumination (700 Lux) for 2 hr. The cercariae released from laboratory-bred snails were also exposed to the three lymnaeid species targeted for observing the metacercariae of E. cinetorchis as described before.
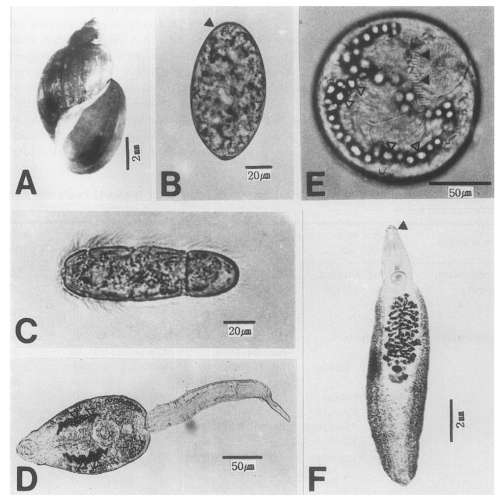
Experimental subjects used in this study. A, A shell of Austropeplea ollula; B, An egg with an inconspicuous operculum (arrow head); C, A miracidium of Echinostoma cinetorchis (× 100); D, A cercaria of Echinostoma cinetorchis. Note the excretory granules; E, A metacercaria of Echinostoma cinetorchis, characterized by the 37-38 spines (black arrow heads) and the excretory granules (white arrow heads); F, An adult worm of Echinostoma cinetorchis. A head crown (arrow head) is noticeable.
RESULTS
Cercarial infectivity of field-collected snails
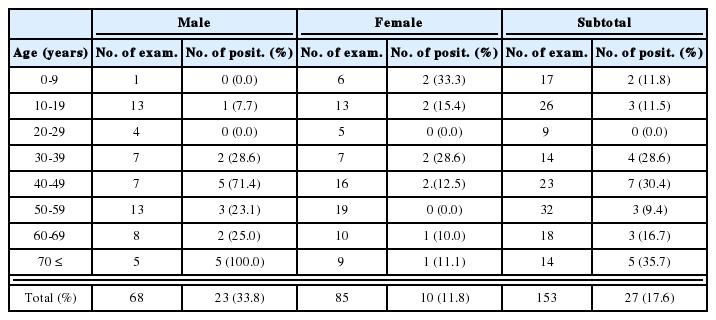
Natural infections of E. cinetorchis in the three lymnaeid snail species collected from several local areas are shown in Table 1. In field-collected lymnaeid snails, only A. ollula (Fig. 1A) was found to be infected with cercariae of E. cinetorchis (infection rate: 0.7%), while none of R. auricularia coreana and F. truncatula were found shedding the cercariae.

Natural infectivity of Echinostoma cinetorchis cercariae in the lymnaeid snails collected from several localities in Korea
The metacercariae of E. cinetorchis were recovered in all S. hemisphaerula (100%) experimentally infected with echinostome cercariae from field-collected Austropeplea snails.
Adult worms were also recovered from the ileo-cecal region of the rats infected with the metacercariae from experimental S. hemisphaerula (Fig. 1F). The recovery rate of 20% was recorded in the rats infected with metacercariae three weeks after metacercarial infection.
Miracidial and cercarial infection to the laboratory-bred snails
When the petri dishes containing embryonated eggs were placed under the light sources (17,000 Lux), almost 90% of the miracidia hatched on the 21-22nd day after incubation at 26℃. Miracidia (Fig. 1C) hatched from eggs of E. cinetorchis were exposed to the laboratory-bred snails of three lymnaeid species suspected as the first intermediate hosts in this study.
Out of 20 snails of each species infected with miracidia, 8 A. ollula and 5 F. truncatula (infection rates, 40% and 25%, respectively) shed a large number of echinostome cercariae (Fig. 1D). The snails of R. auricularia coreana were not infected experimentally with miracidia of E. cinetorchis (Table 2). An average of 1,249 cercariae per each A. ollula snail infected with E. cinetorchis miracidia were released during the 19-day period of observation after the 21st day after miracidial infection (Table 3).
The metacercariae in three laboratory-bred lymnaeid snail species infected with E. cinetorchis cercariae from experimentally infected A. ollula and F. truncatula were also found at the 14th day after cercarial challenge. All three experimental lymnaeid snail species became infected with infection rate of 40%, 100% and 60%, respectively (Table 4 and Fig. 1E).
DISCUSSION
Life-cycle studies of an intestinal trematode, E. cinetorchis in Korea were carried out mainly with planorbid snails (Ahn et al., 1989; Lee et al., 1990; Chung et al., 2001). Out of 3 lymnaeid snail species occurring in Korea, only R. auricularia coreana have been experimentally examined and confirmed that these snails act as the second intermediate host of E. cinetorchis with the metacercarial infection rates of 37-66% (Ahn et al., 1989). However, randomly collected field specimens were all employed for the cercarial susceptibility tests. In the present study, the parasite-free, same-aged, laboratory-bred lymnaeid snails were used for testing their susceptibilities to the E. cinetorchis miracidia and cercariae. Although we observed that R. auricularia coreana were not susceptible to the E. cinetorchis miracidia as confirmed by Ahn et al. (1989), those snails also acted as the second intermediate host of E. cinetorchis with the metacercarial infection rate of 40%.
Lymnaea pervia sharing the breeding niches with H. cantori in Korea was considered to be a possible second intermediate host of E. cinetorchis (Lee et al., 1990). The taxonomic position of this lymnaeid species is confused with that of A. ollula, because Lee et al. (1990) listed L. pervia without any malacological accounts. On the other hand, A. ollula occurring in Korea is very similar to Lymnaea viridis from Australia. Pace (1973) reviewed the taxonomic remarks on A. ollula, mentioning that L. ollula (=A. ollula) and L. pervia are synonyms of L. viridis. Although there is some taxonomic confusion between Lymnaea spp. and A. ollula, Dr. Morrison of the U. S. National Museum mentioned that L. pervia is a junior synonym of L. (=Austropeplea) ollula Gould (Burch et al., 1964). However, we have malacological evidence of Korean populations of A. ollula different from Australian L. viridis. The lymnaeid species employed in this study were identified on the basis of taxonomic keys according to Yoo (1976), Burch et al. (1987) and Kwon (1990). It is the first time that A. ollula was confirmed as the first and second gastropod intermediate hosts of E. cinetorchis in the present study.
Fossaria truncatula, one of the Korean lymnaeid species, is also a possible gastropod to serve as the first and second intermediate hosts of E. cinetorchis based on the experimental results of this study. However, the natural shedding of the E. cinetorchis cercariae was not observed in this study. While two lymnaeid species, R. auricularia coreana and A. ollula, are common mainly in the standing water bodies such as rice fields and pools nationwide in Korea, F. truncatula as an amphibious species is uncommon and with less distribution. Unfortunately, the present authors collected F. truncatula specimens only from a single locality (Sohre, Inchon) because the ecological habitats of F. truncatula were quite limited during the survey period of this study. Therefore, the present authors presume that the natural infection of the E. cinetorchis cercariae from Fossaria snails could be confirmed if more snail populations from various collecting sites are investigated in future studies.
ACKNOWLEDGEMENTS
Authors express their appreciation to Prof. Bernard Fried, Department of Biology, Lafayette College, Easton, Pennsylvania for reading the manuscript.
Notes
This study was supported, in part, by the 2000 Research Fund of Inha University.