Detection of IgG antibody against Neospora caninum in cattle in Korea
Article information
Abstract
A total of 492 cattle sera was screened by IgG-ELISA against Neospora caninum (Nc-1 strain and a Korean isolate, KBA-2) and Toxoplasma gondii. Out of 492, 113 sera (23.0%) reacted positively to either Nc-1 or KBA-2 strains of N. caninum. Among the 113 positive sera, 92 sera (81.4%) reacted with antigens of both strains, but 6 sera (5.3%) with Nc-1 and 15 sera (13.3%) with KBA-2 strain only. And with T. gondii antigen, 6 sera (1.2%) were positive but all reacted with N. caninum antigen also. Western blot revealed typical binding pattern according to ELISA values, such that high OD group reacted specifically to the major surface proteins including 43 kDa protein. Seroprevalence of 23.0% indicates that neosporosis seemed to be one of major causes of abortion in cattle. It is suggested here to establish more epidemiological researches nationwide systematically.
INTRODUCTION
Neospora caninum is a protozoan (Apicomplexa) parasite, first identified by Dubey et al. (1988), causes serious neurological and muscular disease in dogs, cattle, sheep, goats and horses (Dubey and Lindsay, 1993). The life cycle of this parasite was completed by proving dogs as definitive hosts experimentally (McAllister et al., 1998; Lindsay et al., 1999). In recent years, neosporosis has been identified as a major cause of abortion or stillbirth of dairy and beef cattle in many countries (Quintanilla-Gozalo et al., 1999; Davison et al., 1999; Ould-Amrouche et al., 1999; Suteeraparp et al., 1999).
In Korea, N. caninum has been isolated from aborted fetuses of cattle (KBA-1 and KBA-2, Kim et al., 2000). It is necessary to establish more efficient serological diagnostic methods such as ELISA for mass screening of the prevalence of N. caninum infections in cattle. We performed preliminarily IgG-ELISA against the sera of cattle randomly collected from stock farms. On the while, N. caninum shares many features with, but is clearly distinct from, Toxoplasma gondii. They are morphologically and biologically similar in tachyzoite forms and their ubiquitous host ranges. Furthermore, there appears to be serological cross reactivity between N. caninum and T. gondii due to the presence of common antigenic determinants (Bjerkas et al., 1994). Therefore, we tried ELISA against the sera of cattle to antigens of both species.
MATERIALS AND METHODS
Antigen preparation
N. caninum tachyzoites of Nc-1 strain and a Korean isolate (KBA-2, Kim et al., 2000) were maintained in Vero cells (CRL 6318, ATCC, Rockville, MD, USA) in DMEM supplemented with 10% FBS (Gibco BRL Co., Rockville, MD, USA). Pure N. caninum tachyzoites were obtained from supernatant of 3 to 4 days confluent culture. RH tachyzoites of T. gondii were maintained by peritoneal passage in mouse and purified by centrifugation over 40% Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden).
Sera
A total of 492 cattle sera was collected between 1996 and 1998 from stock farms located in mid-to-north parts of Korea and were stored at -70℃ until used. Sex and age of cattle were not identified.
IgG-ELISA
IgG-ELISA was accomplished according to the method of Choi et al. (1992) with some modifications. Briefly, N. caninum and T. gondii extracts were diluted in coating buffer (10 mM sodium phosphate, pH 9.3) at a concentration of 5 µg/ml each and were disposed to 96-well EIA plate (Costar Co., Dover, NH, USA) with 200 µl/well. The plates were incubated overnight at 4℃. After washing with PBS/0.05% Tween-20 (PBS/Tween) three times, 1:100 diluted sera were incubated for 2 hrs at 37℃. After washing with the same buffer, 1:1,000 diluted horse radish peroxidase (HRP)-conjugated goat anti-bovine IgG antibody (Sigma Chemical Co., St. Louis, MO, USA) was added and incubated for 2 hrs. After final washing, 200 µl of substrate solution (1 ml of 1% o-penylene diamine, 50 µl of 30% H2O2 in 99 ml distilled water) was added. The reaction was stopped by adding 20 µl of 5 N H2SO4 after 20 min incubation at 37℃. The absorbance was read at 490 nm with an ELISA reader (Dynatech Lab. Inc., Chantilly, VA, USA).
Western blot
Protein extracts of N. caninum and T. gondii were prepared by ultrasonification in SDS-PAGE sample buffer and were separated by SDS-PAGE and transferred onto nitrocellulose (NC) paper (Schlleicher and Sheull, Keene, NH, USA) (Towbin et al., 1979). NC papers were incubated overnight at 4℃ with blocking buffer (10% heat-inactivated rabbit serum in PBS/Tween). Sera were categorized temporarily with respect to absorbance of ELISA as follow: A, positive OD with both Nc-1 and KBA-2 antigens; B, positive with both but much higher with KBA-2; C, Nc-1 negative but KBA-2 positive; D, Nc-1 positive but KBA-2 negative; and E, positive with Nc-1, KBA-2, and T. gondii, respectively. Sera were applied to NC papers with 1:500 dilution in blocking buffer for 2 hrs at room temperature. After washing three times with PBS/Tween, NC papers were incubated with 1:2,000 diluted HRP-conjugated goat anti-bovine IgG antibody (Sigma Chemical Co.) for 2 hrs in blocking buffer. NC papers were soaked in enhanced chemiluminescence (ECL) solution (Intron Co., Daejeon, Korea) for 1 min and exposed to X-ray film (Konica, Tokyo, Japan) for 5 to 10 sec.
RESULTS
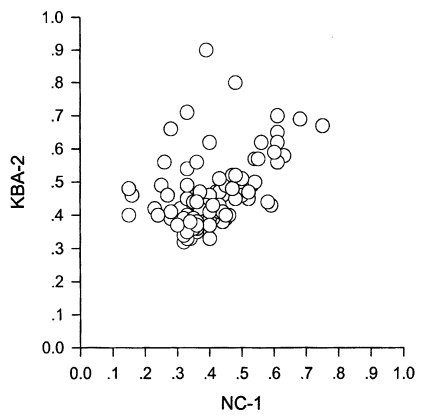
Cut-off values of anti-N. caninum Nc-1 strain, KBA-2 strain, and T. gondii were calculated by the mean + 3SD of optical density in randomly selected 100 sera which were negative both in immunofluorescence assay and western blot. With N. caninum Nc-1 strain antigen, 98 sera out of 492 (19.9%) appeared to be positive with a cut-off value of 0.31 (0.10 ± 0.07). With N. caninum KBA-2 strain antigen, 107 sera (21.7%) were positive by 0.36 (0.15 ± 0.07). Ninety two sera reacted to both Nc-1 and KBA-2 antigens. Overall, 113 sera out of 492 (23.0%) reacted positively to either Nc-1 or KBA-2 strains of N. caninum. And with T. gondii antigen, only 6 sera (1.2%) were positive by 0.30 (0.09 ± 0.07); however, all sera cross-reacted with N. caninum antigen. OD values of all 113 positive sera were distributed separately to compare with OD values of T. gondii as in Fig. 1. When spotted the positives by Nc-1 to KBA-2, they were distributed linearly with some exceptional leaning towards KBA-2 axis as shown in Fig. 2. KBA-2 strain antigen showed much higher specificity than Nc-1 to Korean cattle.
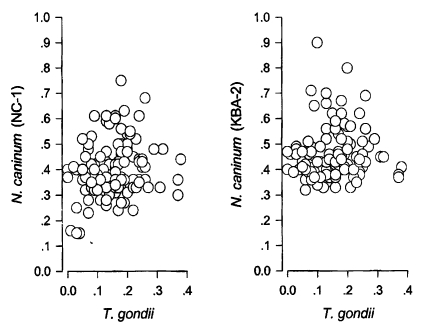
Distribution of positive OD spots of Neospora caninum (Nc-1 and KBA-2 strains) vs. Toxoplasma gondii by IgG-ELISA.
To analyze the binding pattern of positive sera by ELISA, western blot was performed with selected sera from each category as in Fig. 3. Panels A and B showed typical antigenic banding patterns of neosporosis, including major surface membrane antigens of 43, 36, and 29 kDa marked with asterisks (*). In panels C and D, there were no significant bindings to the surface membrane proteins, but the band density to each antigen was equivalent to OD values of sera by ELISA. It was assumed to be reactions with subcellular organellar antigens not defined yet. And in panel E, T. gondii antigens reacted strongly, but did not show specific bindings to the major surface membrane proteins such as 30 kDa (SAG1) and 22 kDa (SAG2) proteins as marked with triangles (Sohn and Nam, 1999).
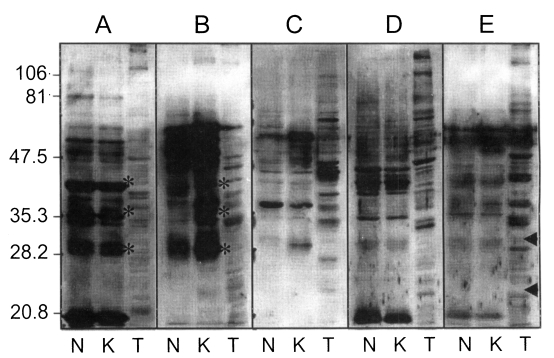
Western blot of sera of various OD values by ELISA. N, Nc-1; K, KBA-2 strain of Neospora caninum; T, Toxoplasma gondii. A, positive with both Nc-1 and KBA-2 antigens; B, positive with both but much higher with KBA-2; C, Nc-1 negative but KBA-2 positive; D, Nc-1 positive but KBA-2 negative; E, positive with Nc-1, KBA-2, and T. gondii.
DISCUSSION
One hundred and thirteen cattle sera out of 492 (23.0%) had positive antibody titers against N. caninum antigens of either Nc-1 or KBA-2 strains by IgG-ELISA. Among the 113 positive sera, 92 sera (81.4%) reacted with antigens of both strains; however, 6 sera (5.3%) reacted only with Nc-1 and 15 sera (13.3%) with KBA-2 only. This indicated that the Korean isolate KBA-2 strain was much more suitable as a diagnostic tool for analysing N. caninum infection in Korea, though there were no differences in morphology of the parasites and pathologic findings in hosts by the infection (Kim et al., 2000). However, Atkinson et al. (1999) reported the difference in biological characteristics between different isolates, which suggested more precise approach to the characterization of KBA-2 strain.
N. caninum shares many features with, but is clearly distinct from, T. gondii. N. caninum had been misdiagnosed as T. gondii up until 1988 largely due to the existence of morphological and biological similarities between tachyzoites of two coccidia and their ubiquitous host ranges. In addition, there appears to be a serological cross reactivity because of shared antigens between N. caninum and T. gondii (Bjerkas et al., 1994). But, they are genetically different and evolve divergently (Marsh et al,, 1995). More clearly, they are different from each other in antigenicity of major surface proteins. For example, 43 (Nc-p43, Hemphill and Gottstein, 1996), 36, and 29 kDa proteins were identified as major surface proteins of N. caninum which were absent in T. gondii and SAG1(p30) and SAG2(p22) of T. gondii are absent in N. caninum (Brindley et al., 1993). Therefore, we tried T. gondii antigen in addition to N. caninum antigens to differentiate the specificity between two species as shown in Fig. 3. In this connection, six sera which reacted to T. gondii antigens seemed not to be T. gondii specific, but a result from cross antigenicities of subcellular organelles such as rhoptry and dense granules between two species. This implied the absence of T. gondii infection in cattle.
Seropositive of 23.0% indicates a high level of prevalence of N. caninum infections in cattle in Korea when compared to other nations (Mainar-Jaime et al., 1999; Suteeraparp et al., 1999), where the neosporosis has emerged and regarded as a major cause of abortion in cattle (Waldner et al., 1999). Neosporosis can cause economic losses in livestock industry (Trees et al., 1999) and the problem can extend to human health and welfare (Tranas et al., 1999; Nam et al., 1998), even though this coccidia has not been found in humans and has no relation to human abortion reported (Petersen et al., 1999) until now. In conclusion, it is strongly suggested that the government or research facilities across the nation need to establish more systematic epidemiological researches nationwide.
Notes
This study was financially supported by the Ministry of Agriculture and Forestry (399002-3).