Age-dependent resistance to Cryptosporidium muris (strain MCR) infection in golden hamsters and mice
Article information
Abstract
An age-dependent aspect of resistance to Cryptosporidium muris (strain MCR) infection was monitored in Syrian golden hamsters, Mesocricetus auratus, at 1-, 5- and 10-week of age and in ICR mice, Mus musculus, at 3-, 12-, and 15-week of age orally inoculated with a single dose of 2×106 oocysts, respectively. The prepatent periods for both animals were similar, independent of age, but the patency was significantly longer in younger hamsters (P<0.001) and a long tendency in younger mice. Hamsters infected at 1-week of age excreted about 10 times higher oocysts than those at 5- and 10-week of age. However, the total oocyst output was similar among mice of different ages. There was a good correlation between the length of the patency and the total oocyst output in hamsters (R=0.9646), but not in mice (R=0.4561). The immunogenicity of the parasite to homologous challenge infections was very strong in hamsters and relatively strong in mice. These results indicate that acquired resistance to C. muris infection is age-related and the innate resistance is independent of age of hamsters, and that both innate and acquired resistance, on the contrary, are irrespective of age of mice.
INTRODUCTION
Cryptosporidium spp. can cause chronic life-threatening diarrhea in neonatal and immunodeficient individuals. In recent years, increasing interest for Cryptosporidium muris infection in cattle has led to the finding of reduced body weight gain and milk production (Anderson, 1987; Esteban and Anderson, 1995). Several transmission experiments to develop laboratory animal model of C. muris have been done in various laboratory animals (Iseki et al., 1989; Aydin, 1991; Matsui et al., 1994; Rhee et al., 1995; Aydin and Özkul, 1996). Meanwhile, age-dependent aspects of resistance to C. parvum and C. baileyi infections were investigated in mice, calves, lambs and chickens, respectively (Sherwood et al., 1982; Heine et al., 1984; Harp et al., 1990; Ortega-Mora and Wright, 1994; Sreter et al., 1995). However, no transmission experiment has been performed, so far, to clarify infectivity of C. muris to golden hamster except for the report of Pavlasek and Lavicka (1995) who revealed a spontaneous gastric cryptosporidiosis from desert hamster (Phodopus roborovskii Satunin, 1903) in the Czech Republic. Although Matsui et al. (1994) described that mice infected with C. muris (strain RN 66) showed a uniform degree of susceptibilities with different ages, experimental evidence in details for age-related resistance to C. muris has not been known in animal models. The present study was to observe the age-dependent resistance to the coccidium in orally infected hamster and mouse by understanding the discharge pattern of oocysts. Additionally, this is the first report which presents the findings of an experimental infection with C. muris (strain MCR) to elucidate basic host-parasite relationship in golden hamsters.
MATERIALS AND METHODS
Ten specific pathogen free (SPF) Syrian golden hamsters at 1-, 5- and 10-week of age and 10 SPF ICR mice at 3-, 12- and 15-week of age were each orally inoculated with a single dose of 2×106 oocysts of C. muris (strain MCR) isolated from mice, respectively, as previously described (Rhee et al., 1995). Each of another five age-matched both animals served as uninoculated controls. The animals were housed as single in wire-bottomed cages and given a commercial nonmedicated concentrates and water ad libitum. Each cage was placed on a tray containing 5 mm depth of water to keep the excrements wet. Five days after oocyst shedding ceased, all the animals were each inoculated again with a single dose of 2×106 oocysts to determine the establishment of a homologous challenge infection. Following inoculation, examination of fecal samples was subjected to the methods previously described by Rhee et al. (1995). Mean values of the lengths of the patent periods in each age for both hosts were compared using Student's t-test. The relationship between the lengths of the patent periods and the total oocyst excretions in each animal was analyzed by linear regression.
RESULTS
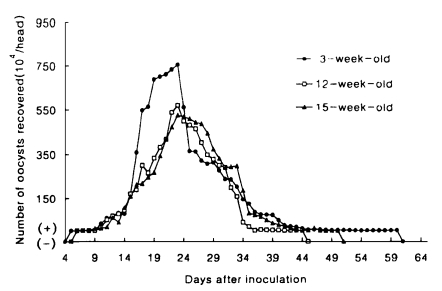
The prepatent periods of C. muris infection in hamsters ranged from 10 to 13 days (Table 1) and in mice from 4 to 6 days (Table 2), irrespective of their ages at inoculation of oocysts. The standard deviation of the mean prepatent period was low for both animals (Tables 1, 2). A significant difference was found in the length of the patent period, ranging from 29.7 to 63.3 days, in hamsters infected at 10-, 5- and 1-week of age (P<0.001), showing an inverse relationship between the age at inoculation and the length of the patent period (Table 1). In contrast to hamsters, a slight difference was found in mice at 12-, 15- and 3-week of age (Table 2).

The length of prepatent and patent period, and the total oocyst excretion of golden hamsters inoculated with 2×106 Cryptosporidium muris (strain MCR) oocysts at different ages
The length of prepatent and patent period, and the total oocyst excretion of mice inoculated with 2×106 Cryptosporidium muris (strain MCR) oocysts at different age
Oocysts were not observed in fecal samples of uninoculated controls. Hamsters infected at 1-week of age excreted a significantly higher number of oocysts (93.4×105, on the average) than those infected at 5- (8.0×105) and 10-week (8.0×105) of age, while mice at 3-, 12- and 15-week of age discharged a similar number of oocysts (from 70.8×106 to 96.3×106). The standard deviations of the means of the patency and those of the total oocyst shedding were high for both animals (Tables 1, 2). The patterns of oocyst excretion in both hosts are depicted in Figs. 1 and 2. Peaks of oocyst productions in hamsters at 1-week of age occurred between day 34 and 43 postinoculation (PI) and in mice at all ages between day 16 and 27 PI, giving rise to one peak. But those in hamsters at 5- and 10-week of age were not prominent. As shown in Tables 1 and 2, the longer its patency, the higher its oocyst excretion was observed in hamsters (R=0.9646), however such relationship was not revealed in mice (R=0.4561).
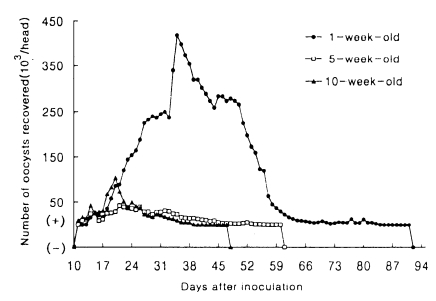
Mean daily oocyst shedding in hamsters inoculated with 2×106 oocysts of Cryptosporidium muris (strain MCR) at 1-, 5- and 10-week of age. (+) and (-) indicate that less than 103 oocysts were detected, and oocysts were not detected, respectively.
Hamsters challenged with oocysts of C. muris did not excrete any oocysts during 30 days post-challenge-infection (PCI), while all mice excreted a few oocysts on day 16 and 31 PCI.
DISCUSSION
The acquisition of age-related resistance to infection with C. parvum has been well characterized in rodent and ruminant models (Sherwood et al., 1982; Harp et al., 1990). However, the age-related aspects of resistance to C. muris were poorly understood in animal models. The present study demonstrates the experimental evidence for the resistance to experimental cryptosporidiosis in hamster.
The prepatent periods of C. muris for both animals were similar irrespective of age, suggesting that the innate resistance to the protozoon is independent of age. Low standard deviation suggests slight individual difference in the innate resistance to C. muris infection for both animals. This is in line with a study that showed a uniform prepatent periods of 7-10 days in mice orally inoculated with C. muris (strain RN 66) at 3 to 29-week of age (Matsui et al., 1994).
In hamster, the older the host, the shorter the patency, suggesting that acquired resistance is age-related in this case, as shown in mice with C. parvum and in chicks with C. baileyi infections (Sherwood et al., 1982; Sreter et al., 1995). However, such phenomenon was scarcely demonstrated in mice, as shown in kids and lambs infected with C. muris (strain MCR) (Rhee et al., 1998).
Interestingly, there was a good correlation between the length of the patent period and the total oocyst excretion in hamsters, but relationship was not shown in mice. High standard deviations of the mean of the patency and those of the total oocyst shedding indicate considerable individual differences in terms of the effectiveness of the acquired resistance to C. muris infection. It is reasonable to think that oocyst production gave rise to one prominent peak in both younger animals due to excretion of oocysts in maximum number, as was found in Cryptosporidium spp. infections.
A single oral infection with C. muris elicited protective immune response of sufficient magnitude to clear the parasite from the gastric mucosa and to make both animals completely resistant to the oral homologous challenge infections. It implies that the immunogenicity of hamsters against C. muris (strain MCR) is very strong even at the age of 1-week.
Based on these experimental results, the acquired resistance to C. muris infection in hamsters is age-related in contrast to mice (possibly independent of age). Additionally, we also revealed that C. muris (strain MCR) is capable of infecting hamsters although a large number of oocyst shedding was not observed in comparison with mice, and hamsters are, therefore, not considered as a favourable experimental animal. All things considered, the mouse has been proven to be the most suitable host of C. muris by a great deal of oocyst shedding in comparison with a small body size and continued infection for a long time.