Toxoplasma gondii antibody titers in sera of children admitted to the Seoul National University Children's Hospital
Article information
Abstract
A total of 542 children under 10 years of age, admitted to the Seoul National University Children's Hospital, was examined for antibody titers of Toxoplasma gondii using indirect latex agglutination (ILA) test. Among them, 7.7% showed positive titers higher than 1:32, without significant difference between males (7.3%) and females (8.5%). The seropositive rate increased with age although the statistical significance was negligible (0.05<P<0.1). By residential areas, the prevalence appeared higher among children from southern provinces (Kyongsang-do and Cholla-do) than those from other areas, but the statistical significance was also very low (0.05<P<0.1). When the seropositive cases were analyzed by coincidental diseases, the prevalence was significantly higher in patients with congenital diseases than in patients with non-congenital diseases (P<0.05). The results showed that the seropositive rate of toxoplasmosis in children examined was not high compared with other endemic countries. Some correlations are suggested between toxoplasmosis and congenital anomalies in Korea.
INTRODUCTION
Toxoplasma gondii is a coccidian protozoa which can cause significant morbidity and mortality in both humans and animals (Dubey and Beattie, 1988). Wolf and Cowen (1937) established T. gondii as a potential cause of neonatal encephalitis in humans, and it was subsequently found that the infection could be congenitally acquired (Paige et al., 1942). Nowadays, the ubiquitous nature of the infection and wide spectrum of clinical manifestations are well known. Especially in immunocompromised hosts such as acquired immunodeficiency syndrome (AIDS), toxoplasmic encephalitis is recognized as an important life-threatening complication (Navia et al., 1986).
For the diagnosis of T. gondii infection, detection of the organism itself is confirmative but very difficult. Thus, most of the clinical laboratories use serological tests to detect antibodies against T. gondii, such as hemagglutination test (HA), indirect latex agglutination test (ILA), enzyme-linked immunosorbent assay (ELISA), and indirect fluorescent antibody test (IFA). The ILA test is most widely used because of its high specificity and high sensitivity (Kobayashi et al., 1978; Balfour et al., 1982).
In Korea, the seroprevalence of T. gondii infection had been reported around 6-14% in the 1960-1970s (Soh et al., 1960; Nakayama et al., 1970), and 2-7% in the 1980-1990s (Choi et al., 1982; Kim and Choi, 1983; Choi et al., 1983, 1985, 1989), showing no much fluctuations during the past 30-40 years. The subjected people were mostly adults from local areas, with or without specific disease backgrounds. However, there have been few reports on the seroprevalence of T. gondii among children including newborns and infants.
In this study, we examined T. gondii antibody titers of 542 children under 10 years of age, who were admitted to the Seoul National University Children's Hospital with various disease backgrounds, using the ILA test. The seropositive cases were compared by various types of coincidental diseases.
MATERIALS AND METHODS
Patients and serum sampling
A total of 542 children under 10 years of age admitted to the Seoul National University Children's Hospital, Seoul, which specialize in the treatment of congenital diseases of children, was randomly selected for this study. Underlying causes of hospitalization, as described in the hospital records, included various types of congenital diseases (n=163) such as malformations of the cardiovascular or musculoskeletal system, and non-congenital diseases (n=379) including neurologic disorders, digestive tract diseases, and neoplasms. Their residential areas were variable; Seoul (n=290), Kyonggi-do (142), Kyongsang-do (37), Cholla-do (24), Kangwon-do (14), and Cheju-do (2). Patients' sera were collected, aliquoted, and frozen to -70℃ until use.
ILA test
The antigen and reagents were purchased from Eiken (Toxotest-MT, Japan). ILA antibody titers were measured according to the published protocols (Kobayashi et al., 1978). Briefly, sera were diluted serially in a U-shaped 96-well microtiter plate and reacted with sensitized latex antigen (Eiken) for 16 hr at room temperature. Antibody titers were determined as the last dilution of sera which precipitated latex in middle class dispersion. Titers of 1:32 or higher were regarded as positive.
Statistical test
The significance of difference between data was tested using the chi-square test. P values lower than 0.05 or 0.1 (if needed) were considered statistically significant.
RESULTS
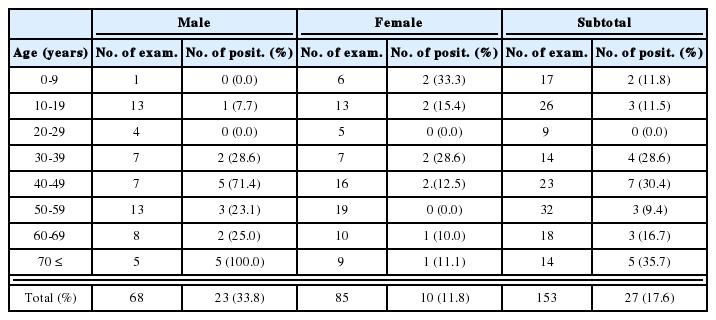
Forty-two (7.7%) of 542 children examined showed positive titers higher than 1:32 by ILA (Table 1). No significant difference in the seropositive rate was observed between males and females; 7.3% (25/341) and 8.5% (17/201), respectively. With respect to age, the prevalence increased with age, although statistical significance was not recognizable (0.05<P<0.1); 3.8% among children under 1 year of age, 7.7% in 1-3 year, 6.4% in 4-6 year, and 12.6% in 7-10 year old children. The seropositive rates were also different by residential areas; higher in southern provinces such as Kyongsang-do (13.5%) and Cholla-do (12.5%) than in Seoul (7.6%), Kyonggi-do (7.0%), Chungchong-do (6.1%), Kangwon-do (0%), and Cheju-do (0%). The difference, however, was statistically not significant (0.05<P<0.1).
When the results were analyzed by coincidental diseases of each case, significant difference was noted between children with congenital diseases (11.7%; 19/163) and that with other kinds of diseases (6.1%; 23/379) (P<0.05) (Table 2). Children with congenital diseases included cardiovascular anomalies (7 cases), musculoskeletal anomalies (5), digestive system anomalies (4), and other congenital diseases (3) (Table 3). Non-congenital diseases were digestive system illness (6), hepatitis with or without tumor (4), dermatologic disorders (4), neurologic disorders (3), heart diseases (3), and ophthalmological problems (3) (Table 4).
Three out of 19 ILA positive children with congenital diseases revealed a very high antibody titer of 1:4,096 (patient codes A-C in Table 3). They were all 9- or 10-year-old males with atrial atresia, heel valgus, or Wilson's disease. Further clinical evaluation of these cases for acute or congenital toxoplasmosis was not performed. The other five cases showed positive ILA titers between 1:64 and 1:512, and they had coincidental diseases of congenital megacolon, pseudoarthrosis, Legg-Calvé-Perthes disease, mitral stenosis, or biliary atresia (patient codes D-H in Table 3).
On the other hand, only one of 23 ILA positive children with non-congenital diseases revealed an antibody titer of 1:4,096 (patient code "a" in Table 3). She had a clinical disease of Henoch-Schönlein's purpura, but there were no signs of acute toxoplasmosis. The other twelve cases with ILA titers between 1:64 and 1:512 had various types of diseases including neurologic disorders in 3 children (patient codes "d", "e", and "j" in Table 4).
DISCUSSION
The present study demonstrated that out of 542 children (under 10 years of age) admitted to a Children's Hospital, T. gondii antibody positive rate was 7.7% by ILA. Seropositive cases were detected among children from various localities, with higher prevalences in southern provinces, Kyongsang-do and Cholla-do, than other areas.
Concerning T. gondii prevalence in Korea, a study performed in 1960 reported a seropositive rate of 5.6% (Soh et al., 1960) and several studies performed during 1970 and 1989 reported seropositive rates of 1.9-14.3% (Nakayama et al., 1970; Choi et al., 1982; Kim and Choi, 1983; Choi et al., 1983, 1985, 1989). Two recent studies on pregnant women by ELISA reported that the seropositive rate was 7.0% (Im et al., 1991) and 4.3% (Ryu et al., 1996). These data and results of the present study together indicate that T. gondii seropositive rate in Korea has not been high compared with other endemic countries such as France, El Salvador, and Austria (Dubey and Beattie, 1988). One of the most responsible factors for the low seropositive rate in Korea is presumed to be the complete cooking of porcine meat. The distribution of few wild animals in the environment might also be another factor.
It is generally known that T. gondii prevalence is not significantly different between males and females (Beverley et al., 1976), which the present study confirmed. However, it is acknowledged that the seroprevalence increases with age as shown in data from various countries (Dubey and Beattie, 1988). In North America an upsurge of prevalence was noted during adolescent ages, and in Central and South America there was a steady rise in prevalence during childhood (Feldman and Miller, 1956; Fleck, 1969). In this study, a higher seroprevalence was observed among 7-10 year-old children than in younger children.
When pregnant women acquire primary infection with T. gondii especially during their second or third trimester, they can transmit the infection transplacentally to their fetus (Beaver et al., 1984). The infected babies may develop congenital toxoplasmosis and suffer from symptoms such as chorioretinitis, convulsions, jaundice, hydrocephalus, fever, pneumonitis, hepatosplenomegaly, lymphadenopathy, microcephalus, cataract, hypothermia, and rash (Sever et al., 1988). However, up to 75% of the infected babies have no clinical manifestations (Marjaleena et al., 1989), and most toxoplasmic children develop clinical manifestations several years after birth (Desmontz and Thulliez, 1985). In this study, it is regreted that maternal sera were not examined and whether seropositive cases were infected congenitally or not was unclear. However, at least some of the seropositive cases are considered to have been infected congenitally.
It is of particular interest that a significantly higher seropositive rate was observed among children with congenital diseases than those with other causes of hospitalization. The major congenital diseases involved were developmental anomalies of the cardiovascular, musculoskeletal, digestive or urinary system. Relatively scarce information has been available on the relationship between T. gondii infection and congenital anomalies. A high risk of children to have an anomaly of the diaphragm or specific cardiovascular anomalies was reported among children born to mothers with high T. gondii antibody titers (Sever et al., 1988). Coincidence of congenital toxoplasmosis and biliary atresia was reported in a 4-week-old infant (Glassman et al., 1991). Congenital developmental anomalies of the visual system in relation with toxoplasmosis were also reported (Lukasik-Czerek, 1990).
The reason for a significant correlation between congenital anomalies and congenital T. gondii infection is yet unclear. However, it can be interpreted that the children with congenital diseases are at a high risk of getting infectious diseases such as toxoplasmosis during the perinatal period. However, any adverse effects of congenital T. gondii infection on the development of fetal organs during the gestational period should be further studied and clarified.
Three children aged 9-10 with atrial atresia, heel valgus, or Wilson's disease, and one child with Henoch-Schönlein's purpura revealed a very high antibody titer of 1:4,096. Since a further clinical evaluation was not performed on these cases, it is unclear whether they had symptoms of acute toxoplasmosis or not. Three children (two 3-year-old males and one 9-year-old female), with antibody titers of 1:64 or 1:128, who complained of neurologic disorders such as cerebral palsy and spastic paraplegia may have suffered from congenital toxoplasmosis.
Notes
This study was supported by Research Grant No. 94-1400-08-01-3 of The Korea Science and Engineering Foundation (1994).