Abstract
Toxoplasma gondii Korean isolate (KI-1) tachyzoites were inoculated intraduodenally to BALB/c mice using a silicon tube, and the course of infection and immune responses of mice were studied. Whereas control mice, that were infected intraperitoneally, died within day 7 post-infection (PI), the intraduodenally infected mice survived until day 9 PI (infection with 1×105 tachyzoites) or day 11 PI (with 1×106 tachyzoites). Based on histopathologic (Giemsa stain) and PCR (B1 gene) studies, it was suggested that tachyzoites, after entering the small intestine, invaded into endothelial cells, divided there, and propagated to other organs. PCR appeared to be more sensitive than histopathology to detect infected organs and tissues. The organisms spread over multiple organs by day 6 PI. However, proliferative responses of splenocytes and mesenteric lymph node (MLN) cells in response to con A or Toxoplasma lysate antigen decreased significantly, suggesting immunosuppression. Splenic CD4+ and CD8+ T-lymphocytes showed decreases in number until day 9 PI, whereas IFN-γ and IL-10 decreased slightly at day 6 PI and returned to normal levels by day 9 PI. No TNF-α was detected throughout the experimental period. The results showed that intraduodenal infection with KI-1 tachyzoites was successful but did not elicit significant mucosal immunity in mice and allowed dissemination of T. gondii organisms to systemic organs. The immunosuppression of mice included reduced lymphoproliferative responses to splenocytes and MLN cells to mitogen and low production of cytokines, such as IFN-γ, TNF-α, and IL-10, in response to T. gondii infection.
INTRODUCTION
Toxoplasma gondii is an intracellular protozoan that can cause significant morbidity and mortality in humans and animals [1]. Cats and foxes are the natural definitive hosts, and various other animals, including humans and mice, serve as intermediate hosts. T. gondii has several developmental stages, including rapidly dividing tachyzoites, slowly proliferating bradyzoites, and oocysts containing sporozoites [2]. Bradyzoites and tachyzoites in tissue cysts and sporozoites in oocysts are infective to intermediate and definitive hosts. Freed, extracellular tachyzoites can be transmitted mouse-to-mouse by peritoneal injection and continuously maintained in the laboratory. However, extracellular tachyzoites are fragile to gastric acid and HCl, and thus oral infection with freed tachyzoites is less likely to occur [3-5].
Strains and isolates of T. gondii are classified into 3 genotypes (I, II, and III) according to the restriction fragment length polymorphism (RFLP) patterns [6,7]. The virulent type I strain is most frequently seen in congenital toxoplasmosis of humans, whereas the type II predominates in human adult toxoplasmosis (including AIDS patients), and the type III is common in wild animal infections [6]. The Korean isolate KI-1, which was isolated from blood of an ocular patient, is similar to the RH strain in various aspects, i.e., morphology, virulence, pathogenicity, tissue culture characteristics, and gene sequences [8,9]. However, more studies are needed to determine the characteristics of KI-1 isolate, including its immunological properties.
Bradyzoites and oocysts, when orally introduced, encounter non-immunologic host defenses, including gut motility, epithelial cell layer, gastric acid, and mucus in the stomach [10]. Their cyst walls are dissolved by gastric acid and proteolytic enzymes, and the released parasites invade the epithelial cells or enterocytes of the small intestine and disseminate into deep tissues according to the parasite motility and virulence [10]. When enterocytes are infected by the parasites, they can play a role as antigen presenting cells (APC) and secrete cytotoxic molecules (e.g. nitric oxide), chemokines (e.g. IFN-γ inducible proteins), and cytokines (e.g. IL-1, IL-6, and GM-CSF) [10,11]. The chemokines and cytokines attract innate immune cells, and the stimulated immune cells produce IL-12. IL-12 then stimulates T cells and NK cells to secrete IFN-γ which, in turn, activates innate immune cells for parasite clearance and thus damages the intestinal integrity. In addtion, lamina propria CD4+ T-lymphocytes produce increased quantities of IFN-γ and TNF-α and participate in the host defense [10,11]. To stop parasite multiplication and to prevent host death due to T. gondii infection, IL-12 and IFN-γ are required [12].
To observe the host immune responses against T. gondii KI-1 infection, it is pre-requisite to determine the possibility and course of infection in different organs and tissues after peroral infection. However, tachyzoites are fragile to gastric acid and HCl and simple oral infection with KI-1 tachyzoites may be failed. Therefore, the present study aimed to determine whether it is possible to infect mice by intraduodenal inoculation of KI-1 tachyzoites. The presence of parasites in organs and tissues of mice were determined histopathologically and by detection of the B1 gene, and mucosal and systemic immune responses were observed.
MATERIALS AND METHODSExperimental infection of miceBALB/c mice, 6-8 weeks old, purchased from SPF animal center (Koatech Company, Kyonggi-do, Republic of Korea) were used. Korean isolate-1 (KI-1) of T. gondii has been maintained by intraperitoneal injection of BALB/c mice [8]. Mice were starved for food for 1 day and then orally inoculated with 105 or 106 tachyzoites in 0.2 ml PBS using a narrow silicon tube inserted into the duodenum passing through the stomach. Control mice received 0.2 ml PBS without tachyzoites. For exact intraduodenal injection, it was confirmed that the whole length of silicon tube was properly intubated into the duodenum. Possibility for the tip of the tube remaining in the stomach could be ruled out by repeated preliminary practices. The introduction of this silicon tube was performed carefully to avoid any damage to the esophagus or stomach.
Each experimental group consisted of 6 mice. To confirm infection, 3 infected mice per group, were sacrificed under ethyl ether anesthesia, and the brain, heart, liver, lung, stomach, duodenum, jejunum, kidney, spleen, and mesenteric lymph nodes (MLN) were collected on days 2, 4, 6, and 8 after infection.
Histopathologic examinationsTo examine the infection status of visceral organs after intraduodenal inoculation, the brain, heart, liver, lung, stomach, duodenum, jejunum, ileum, kidney, and spleen of mice were removed. These organs were fixed in 10% neutral formalin and embedded in paraffin. The embedded sections were cut into a thickness of 4 µm, stained with Giemsa, and examined for tachyzoites under a light microscope.
PCR analysis for T. gondii B1 geneTo genetically identify the presence of KI-1 tachyzoites in visceral organs, PCR analysis was performed to detect Toxoplasma B1 gene as a diagnostic gene [13]. DNA extraction was performed using the DNeasy®Tissue kit (Qiagen, Hilden, Germany). Forward- and reverse-primers were: 5'-AAA AAT GTG GGA ATG AAA GAG-3' and 5'-ACG AAT CAA CGG AAC TGT AAT-3', respectively. Amplification of the B1 gene was completed in the following conditions: 1 cycle of 5 min at 95℃ for initial denaturation followed by 30 cycles of 1 min at 95℃, 1 min at 60℃, and 3 min at 74℃. Amplification was performed using a DNA thermal cycler (GeneAmp™, PCR system 9600, PE Applied Biosystems, Foster City, California, USA). PCR amplification products were examined in 1.5% agarose gels and confirmed by staining with ethidium bromide and visualized under UV.
Survival of miceSix-week-old female BALB/c mice were inoculated with 105 or 106 T. gondii KI-1 tachyzoites into the duodenum using a silicon tube. The survival time of mice was recorded everyday post-infection (PI).
Preparation of Toxoplasma lysate antigen (TLA)
T. gondii KI-1 tachyzoites were maintained by serial passage in BALB/c mice. Tachyzoites were inoculated intraperitoneally and harvested by peritoneal lavage on day 5 or 6 PI. The peritoneal exudate was washed with sterile PBS and pelleted by centrifugation for 10 min at 3,000 rpm [14]. Tachyzoites were purified using 40% Percoll (Pharmacia Biotech, Uppsala, Sweden) in PBS. The purified tachyzoites were disrupted by 5 cycles of freezing at -70℃ and thawing at room temperature. The homogenates were centrifuged at 12,000 rpm at 4℃ for 30 min, and the supernatant was used as the antigen after filtration through a 0.45 µm membrane (Advantec MFS Inc., Pleasanton, California, USA).
Lymphocyte preparationThe spleen and MLN were removed aseptically from the infected and uninfected control mice and kept in cold RPMI 1640 (Gibco BRL, Grand Island, New York, USA) containing 10% heat-inactivated FBS (Gibco BRL), 10 mM HEPES, 2 mM L-glutamine, 2 mM sodium bicarbonate, 5×10-5 M 2-mercaptoethanol (2-ME), and antibiotics (100 IU/ml of penicillin G and 100 µg/ml of streptomycin) (Gibco BRL). Tissues were washed with RPMI 1640 containing 2% FBS and prepared to a single cell suspension [15]. After RBC lysis using a hypotonic buffer containing NH4Cl, the single cell suspensions of MLN and spleen were washed 3 times by centrifugation. Finally, cells were resuspended in complete RPMI 1640 supplemented with 10% FBS, and viable cells were counted by trypan blue exclusion test. Concanavalin A (con A) (Sigma-Aldrich, St. Louis, Missouri, USA) was added at a predetermined final concentration of 5 µg/ml.
Fluorescence-activated cell sorting (FACS)Single cell suspensions of the spleen and MLN were prepared as described above. Cell suspensions were adjusted to 1×107 cells/ml with complete RPMI, and 100 µl (1×106 cells) of the cell suspension was then used for FACS staining. To avoid non-specific binding of fluorescein-conjugated antibody, Fc blocker (anti-CD16/32 [Fcγ III/II receptor]) (eBioscience, Boston, Massachusetts, USA) was used before labeling specific antibodies with anti-mouse CD3, CD4, CD8, DX5, or F4/80. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (eBioscience), phycoerythrin (PE)-conjugated anti-CD4 (eBioscience), PE-conjugated anti-DX5 (eBioscience), cyanin 5-phycoerythrin (Cy5-PE)-conjugated anti-CD8 (eBioscience), and Cy5-PE-conjugated anti-F4/80 (eBioscience). After staining, the cells were washed with 2% FBS/HBSS and then fixed with 2% paraformaldehyde. Phenotypic analysis of CD4+- or CD8+-T cells, NK cells, and macrophages was performed by FACScan flow cytometer (Becton-Dickinson, Rutherford, New Jersey, USA). Gates were set to exclude nonviable cells and adjusted to detect specifically stained cells. Data are presented by the percentage of positive cells.
T-cell proliferation assayThe spleen and MLN were removed aseptically from infected mice at days 2, 4, and 6 PI. Single cell suspensions were prepared and adjusted to 1×107 cells/ml with complete RPMI, and 40 µl (4×105 cells) was used for the cell proliferation assay. The assays were carried out in a 96-well flat-bottomed plate (Nalge Nunc Int., New York, USA) in triplicate in 0.2 ml volumes with following conditions: 4×105 cells per well in 0.2 ml of culture media alone or in media containing 100 µg/ml Toxoplasma KI-1 lysate antigen (TLA) or 5 µg/ml con A. Plates were incubated for 72 hr in 5% CO2 at 37℃ and viable cells were converted to a colored product by XTT solution, i.e., 2,3-Bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (Roche Diagnostics GmbH, Mannheim, Germany). Briefly, a mixture of 1 µl phenazine methosulphate (PMS; electron-coupling reagent) and 50 µl of the XTT labeling reagent were added to each well and incubated at 37℃ during the last 4 hr of culture. Absorbance was read at 450 nm with a reference wavelength of 650 nm (ELx808, BioTek Instruments, Winooski, Vermont, USA). Average absorbance values were obtained from the net absorbance values, i.e., absorbance of seeded wells minus absorbance of blank wells.
RT-PCR for detection of cytokinesThe spleen was removed at days 0, 6, and 9 PI to examine cytokine signals. The total RNA was extracted by homogenization of the spleen in TRIzol Reagent (Invitrogen, Calsbad, California, USA) according to the manufacturer's protocol. After adding chloroform (20% of the total volume), the mixture was shaken for 15 sec, incubated at 15-30℃ for 2-3 min, and then centrifuged at 12,000 g for 15 min at 4℃. An equal volume of isoprophyl alcohol was added, and the mixture was incubated at room temperature for 10 min. After centrifugation at 12,000 g for 10 min at 4℃, the pellet was washed with 75% ethanol in diethyl pyrocarbonate (DEPC) water, air-dried and dissolved in an appropriate volume of DEPC water for cDNA preparation.
Reverse transcription for the first strand DNA was performed using the Superscript™III First-Strand Synthesis system for RT-PCR (Invitrogen). Briefly, total RNA (2 µg) was added to reaction mixture containing 10 mM dNTPs and Oligo (dT), incubated at 65℃ for 5 min, and then placed on ice for 1 min. It was then added to the mixture containing SuperScript™III RT, and incubated at 50℃ for 50 min and 85℃ for 5 min. The cDNA was amplified with each cytokine primer by GeneAmp PCR system 9600 (PE Applied Biosystems, Foster City, California, USA). The primers for mouse cytokines (IL-10, IFN-γ, TNF-α, and 18s RNA) were prepared: IL-10; sense: 5'-CGG GAA GAC AAT AAC TG-3'; anti-sense: 5'-CAT TTC CGA TAA GGC TTG G-3'; IFN-γ; sense: 5'-AAC GCT ACA CAC TGC ATC TTG G-3'; anti-sense: 5'-GAC TTC AAA GAG TCT GAG G-3'; TNF-α; sense: 5'-GAT CTC AAA GAC AAC CAA CTA GTG-3'; anti-sense: 5'-CTC CAG CTG GAA GAC TCC TCC CAG-3'; 18s RNA sense: 5'-CGC GGT TCT ATT TTG TTG GT-3'; anti-sense: 5'-AGT CGG CAT CGT TTA TGG TC-3'. There were 35 PCR cycles for all cytokines, except for TNF-α which had 32 cycles. Annealing temperatures were set for IL-10 (48℃), IFN-γ (48℃), TNF-α (55℃), and 18s rRNA (60℃). The amplified PCR products were electrophoresed in 2% agarose gel and photographed under UV light after ethidium bromide stain. The density of each band was analyzed using the densitometrical analysis program, TINA (Raytest, Straubenhardt, Germany). The optical densities of the bands were normalized by the housekeeping gene, 18s rRNA, and the quantity of naive mRNA was calculated. When the value of IFN-γ/18s RNA of uninfected mice was "1" (standard value), the values of IFN-γ/18s RNA of infected mice were presented as a fold change.
RESULTSSurvival of miceThe virulence of T. gondii KI-1 tachyzoites was estimated by the survival of intraduodenally infected mice (Fig. 1). At the inoculum dose of 105 tachyzoites, 4 (66.7%) of 6 infected mice lived until day 10 PI, and all mice died by day 11 PI. At the dose of 106 tachyzoites, 4 (66.7%) of 6 infected mice lived until day 8 PI, and all mice died by day 9 PI (Fig. 1).
Detection of tachyzoites from organs and tissuesThe presence of KI-1 tachyzoites in organs and tissues of infected mice was determined by light microscopy of histopathological slides (Table 1; Fig. 2) and by PCR using the B1 gene primer (Fig. 3). At the inoculum dose of 105 tachyzoites, tissue samples stained with Giemsa revealed T. gondii tachyzoites in the lungs and spleen at day 6 PI (Table 1; Fig. 2). In mice inoculated with 106 tachyzoites, tachyzoites were seen in sections of the liver and spleen at day 8 PI (Table 1). However, PCR appeared to be more sensitive than histopathology in detecting tachyzoites. In mice inoculated with 105 tachyzoites, the B1 gene was detected in the liver, lungs, stomach, kidney, MLN, and spleen on day 6 or 8 PI (Table 1). In mice inoculated with 106 tachyzoites, the B1 gene was detected in the duodenum and ileum as early as day 2 PI, and they continued to be detected in the duodenum on days 4, 6, and 8 PI (Table 1). The B1 gene was also expressed in the liver, jejunum, kidney, MLN, and spleen at day 8 PI. In both inoculum doses, there was no evidence of tachyzoite invasion into the brain in this experimental setting.
Proliferative responses of splenocytes and MLN cellsWhen splenocytes and MLN cells were harvested and stimulated with mitogen (con A) or Toxoplasma lysate antigen (TLA), the absorbance of con A-treated splenocytes was high at days 0 and 3 PI, and then gradually decreased until day 9 PI (Fig. 4). The absorbance of TLA-treated splenocytes was low at day 0 PI, increased in the early stage of infection (days 3 and 6 PI), and then decreased in the late stage of infection, with mortality of infected mice (Fig. 4). The proliferative responses of MLN cells were high by stimulation with con A at day 0 PI but became low during days 3-9 PI, suggesting immunosuppression (Fig. 4).
Phenotype changes of splenocytes and MLN cellsAmong the splenocytes and MLN cells from the infected mice, the proportions of NK cells and macrophages did not change significantly throughout the experimental period (Fig. 5). However, in the spleen, the proportions of CD4+ T-cells and CD8+ T-cells decreased through the experimental period. In the MLN cells, similar decreases of CD4+ T-cells were seen. However, CD8+ T-cells almost maintained their proportions throughout the experimental period (Fig. 5).
Cytokine levels in splenocyte culturesThe level of IFN-γ in the spleen of T. gondii KI-1 tachyzoite-infected mice decreased 0.6 fold compared to normal uninfected mice at day 6 PI and increased 1.45 fold at day 9 PI although the difference was not statistically different (P>0.05). The level of TNF-α was similar between the infected and uninfected mice (data not shown). IL-10 decreased 0.57 fold at day 3 PI and returned to the normal value by day 9 PI (Fig. 6).
DISCUSSIONTo investigate the virulence of Toxoplasma, survival of mice after intraperitoneal injection with tachyzoites is measured [16,17]. However, this experimental infection route and the parasite stage involved are different from those in natural human infections. In humans, the main mode of infection is oral intake of undercooked meat or fecal inoculum. The course of infection and tissue spreading of Toxoplasma, as well as immune responses of the host, may differ between intraperitoneal and oral infections and beween tachyzoite and bradyzoite/oocyst infection. Tachyzoites are fragile to gastric acid, and oral infection with tachyzoites is less possible. Therefore, in the present study, an intraduodenal inoculation method that insert a silicon tube into the duodenum was tried to infect mice. The course of infection and immunological responses of the mice were then investigated.
Researchers reported that bradyzoites are resistant to gastric digestion and remain orally infective whereas tachyzoites are destroyed by gastric juice within 1 hr [3-5]. However, Dubey [18] reported that tachyzoites occasionally survived at acid peptic digestion for 2 hr, and the strain of T. gondii did not affect the susceptibility of tachyzoites to acid pepsin solution. Extracellular tachyzoites were infective to mice and cats if orally inoculated, although the infectivity was dose-dependent [18]. He [18] judged the infectivity by observing survival of mice. In our study, the organisms and their B1 gene were detected in various organs of mice. These results showed successful intraduodenal infection, although some inoculated tachyzoites may have been regurgitated to the stomach and underwent death.
We could not use bradyzoites because KI-1 is highly virulent [8] and it is difficult to make tissue cysts in mice. In the RH strain, tissue cysts were successfully produced at day 45 PI by subcutaneous injection of 105 tachyzoites in mice treated with sulfadiazine and NaHCO3 [19]. However, these tissue cysts failed to infect mice via the oral route probably due to their incomplete conversion to bradyzoites [19]. It is unknown whether KI-1 could produce tissue cysts like the RH strain.
The B1 gene of KI-1 was detected at earlier stages in the small intestine and in later stages in multiple organs, including the liver, spleen, kidneys, and lungs, which clearly indicate rapid systemic spreading of tachyzoites. However, the results of PCR, in terms of the kinds of organs in which the B1 gene was detected and the time when it was first detected, varied by the inoculum dose, 105 or 106, and also by individual mouse. It is difficult to explain why the B1 gene was not expressed in the lungs of 106 tachyzoite-inoculated mice, although it was strongly expressed in 105 inoculated mice during days 6-8 PI. It is also uncertain why the B1 gene was not expressed in MLN of 106 inoculated mice, although it was expressed in 105 inoculated mice at day 8 PI. Further studies on the chronologic spreading of tachyzoites in mice are needed.
When RH strain is injected intraperitoneally to mice, tachyzoites multiply rapidly, disseminate systemically, and reach lethal burdens (up to 106 parasites/g tissue) [20]. Lethality is characterized with excessive levels of Th1 cytokines, particularly IL-12, IL-18, and IFN-γ, which paradoxically are required for the protection of hosts [20]. Early stage interactions between parasites and innate immune responses of the host seemed to be critical in triggering the lethal cascade of cytokines [20]. Our study suggested that intraduodenally inoculated KI-1 tachyzoites remained for a short time in the small intestine and then disseminated systemically. Therefore, innate immune responses of mice may have been less drastically stimulated. Consequently, cytotoxic inflammation and immunity appreared slowly, followed by immunosuppression as a common result of toxoplasmosis. For this reason, the lethal time in our study may have been delayed a little compared to intraperitoneal inoculation [8]. All intraduodenally infected mice survived for 9 days when infected with a dose of 105, whereas the survival time was only 4 days in intraperitoneally infected mice [8]. On the other hand, the survival time was 7 days in intraduodenally infected mice, but 3 days in intraperitoneally infected mice with the infection dose of 106 [8].
When a virulent T. gondii invades the epithelial cells of the intestine, it can induce a severe form of intestinal inflammation, including loss of intestinal epithelial architecture, shortened villi, massive influx of inflammatory cells into the lamina propria, and scattered patches of necrosis [10]. Non-specific immune responses are then triggered, and IL-12 or IFN-γ are produced from activated NK cells and macrophages. These cells then promote specific acquired immune responses, involving CD4+ and CD8+ T-lymphocytes, and damage the intestinal integrity [21-23]. However, in our experiment, NK cells and macrophages, the innate immune cells, did not increase among the splenocytes and MLN cells. They remained at control levels during the 9 days infection period. In addition, CD4+ and CD8+ T-lymphocytes decreased among the splenocytes and MLN cells with a reduction in lymphoproliferative capacity to mitogen (con A) or T. gondii antigens (TLA) stimulation. These results indicate that the host immune system had been destroyed and lymphopenia ensued. In addition, IFN-γ and IL-10 production by splenocytes decreased at day 6 PI. These results together suggest that intraduodenal inoculation of KI-1 tachyzoites induced immunosuppression as a whole, ultimately resulting in reduction of Th1 responses, followed by death of mice.
There are few reports on oral infection of T. gondii tachyzoites. Hence, the splenic T-cell immune responses in our study could be compared with a study on oral inoculation of bradyzoites [24]. After an oral inoculation with bradyzoites (76K strain, 40 cysts), T. gondii was found within 24 hr in the spleen [24]. NK 1.1+ cells increased from day 1 to day 10 PI, whereas CD4+ and CD8+ T-cells decreased until day 7 PI and significantly increased thereafter [24]. IFN-γ production increased continuously from infection, and IL-4 and IL-10 increased after 4 days [24]. These results have similiarities with our study. However, the detection time of tachyzoites in our study was later (day 6 PI) in the spleen after infection with 105 tachyzoites. This difference may have been due to the parasite strain, size of inoculum, infection route, mouse stain, and different method of parasite detection. Oral infection with T. gondii bradyzoites stimulated strong cellular immune responses that polarized toward an early Th1 response and a late Th2 response [24]. However, in our study, intraduodenal inoculation disseminated parasites slowly and suppressed adoptive host immunity, including CD4+ and CD8+ T-cell responses. Also, IFN-γ level was not elevated and lymphopenia appeared, which may have permitted growth and multiplication of tachyzoites and induced host death.
ACKNOWLEDGEMENTSThis research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Ministry of Education, Science and Technology (No. 2009-0076262).
REFERENCES1. Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the Unites States. Int J Parasitol 2008;38:1257-1278. PMID: 18508057.
2. Cenci-Goga BT, Rossitto RV, Sechi P, McCrindle CME, Cullor JS. Toxoplasma in animals, food, and humans: an old parasite of new concern. Foodborne Pathog Dis 2011;8:1-12. PMID: 21043837.
3. Pettersen EK. Destruction of Toxoplasma gondii by HCl solution. Acta Pathol Microbiol Scand B 1979;87:217-220. PMID: 495099.
4. Sharma SP, Dubey JP. Quantitative survival of Toxoplasma gondii tachyzoites and bradyzoites in pepsin and in trypsin solutions. Am J Vet Res 1981;42:128-130. PMID: 6784616.
5. Popiel I, Gold MC, Booth KS. Quantification of Toxoplasma gondii bradyzoites. J Parasitol 1996;82:330-332. PMID: 8604108.
6. Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 1995;172:1561-1566. PMID: 7594717.
7. Howe DK, Honoré S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 1997;35:1411-1414. PMID: 9163454.
8. Chai JY, Lin A, Shin EH, Oh MD, Han ET, Nan HW, Lee SH. Laboratory passage and characterization of an isolate of Toxoplasma gondii from an ocular patient in Korea. Korean J Parasitol 2003;41:147-154. PMID: 12972728.
9. Lin A, Shin EH, Kim TY, Park JH, Guk SM, Chai JY. Genetic characteristics of the Korean isolate KI-1 of Toxoplasma gondii. Korean J Parasitol 2005;43:27-32. PMID: 15793356.
10. Buzoni-Gatel D, Schultíhess J, Menard LC, Kasper LH. Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell Microbiol 2006;8:535-544. PMID: 16548880.
11. Mennechet FJD, Kasper LH, Rachinel N, Li W, Vandewalle A, Buzoni-Gatel D. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol 2002;168:2988-2996. PMID: 11884471.
12. Nguyen TD, Bigaignon G, Markine-Goriaynoff D, Heremans H, Nguyen TN, Warnier G, Delmee M, Warny M, Wolf SF, Uyttenhove C, Van Snick J, Coutelier JP. Virulent Toxoplasma gondii strain RH promotes T-cell-independent overproduction of proinflammatory cytokines IL 12 and γ-interferon. J Med Microbiol 2003;52:869-876. PMID: 12972580.
13. Cabaňas-Cortés MA, Reyes-Maldonado E, Montiel-Cervantes L, Domínguez-López ML, Jiménez-Zamudio L, García-Latorre E. Toxoplasma gondii: effect of maternal infection in the development of lymphoid organs of BALB/c neonates. Exp Parasitol 2009;121:279-287. PMID: 19114038.
14. Shin EH, Kim SB, Nam HW, Han ET, Park JH, Ahn HJ, Chai JY. Use of monoclonal antibodies for flow cytometric detection of intracellular Toxoplasma gondii in murine splenic lymphocytes. J Parasitol 2004;90:161-166. PMID: 15040684.
15. Shin EH, Lee SH, Kim JL, Park YK, Chai JY. T-helper-1 and T-helper-2 immune responses in mice infected with the intestinal fluke Neodiplostomum seoulense: their possible roles in worm expulsion and host fatality. J Parasitol 2007;93:1036-1045. PMID: 18163337.
16. Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol 1993;151:1430-1440. PMID: 8335939.
17. Guk SM, Kook J, Jeon YH, Choi JH, Han ET, Shin EH, Chai JY. Suppressed cytokine and immunoglobulin secretions by murine splenic lymphocytes infected in vitro with Toxoplasma gondii tachyzoites. J Parasitol 2005;91:467-470. PMID: 15986629.
18. Dubey JP. Re-examination of resistance of Toxoplasma gondii tachyzoites and bradyzoites to pepsin and trypsin digestion. Parasitology 1998;116:43-50. PMID: 9481773.
19. Villard O, Candolfi E, Ferguson DJP, Marcellin L, Kien T. Loss of oral infectivity of tissue cysts of Toxoplasma gondii RH strain to outbred Swiss Webster mice. Int J Parasitol 1997;27:1555-1559. PMID: 9467741.
20. Mordue DG, Monroy F, Regina ML, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 2001;167:4574-4584. PMID: 11591786.
21. Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-γ-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med 1996;184:597-607. PMID: 8760813.
22. Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA 1993;90:6115-6119. PMID: 8100999.
23. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 1988;240:516-518. PMID: 3128869.
24. Lee YH, Channon JY, Matsuura T, Schwartzman JD, Shin DW, Kasper LH. Functional and qunatitative analysis of splenic T cell immune responses following oral Toxoplasma gondii infection in mice. Exp Parasitol 1999;91:212-221. PMID: 10072323.
Fig. 1Survival of mice infected intraduodenally with T. gondii KI-1 tachyzoites. At the inoculum dose of 106 tachyzoites per mouse, 66.7% survived until day 8 PI and all mice died by day 9 PI. At the inoculum dose of 105 tachyzoites, 66.7% survived to day 10 PI and all mice died by day 11 PI. 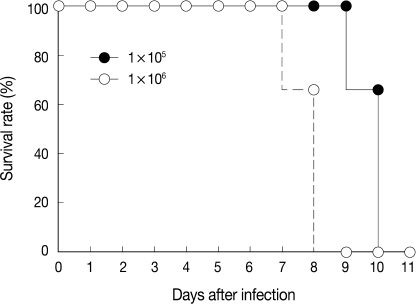
Fig. 2Section of the mouse lung showing a T. gondii KI-1 tachyzoite (arrow) at day 6 PI with 105 tachyzoites. Giemsa stain. ×1,000. 
Fig. 3PCR analysis with B1 gene (469 bp) primer of T. gondii from day 2 to day 8 PI with the infection dose of 105 (left) and 106 (right) tachyzoites. Lane M is 1 kb DNA ladder. DNA of KI-1 was detected in the liver, lungs, stomach, kidney, mesenteric lymph node (MLN), and spleen at day 8 PI with an inoculum dose of 105. When the inoculum dose was 106 tachyzoites, DNA of KI-1 was detected in the liver, duodenum, jejunum, MLN, and spleen at day 8 PI. P: positive control, DNA from a mouse infected with KI-1, N: negative control, DNA from a normal mouse. 
Fig. 4Proliferative responses of splenocytes (left) and mesenteric lymph node (MLN) cells (right) from mice infected with 106 T. gondii KI-1 tachyzoites incubated alone or stimulated with con A or T. gondii lysate antigen (TLA) as measured by the colorimetric XTT assay. The absorbance of con A-treated splenocytes decreased gradually until day 9 PI which suggests suppression of immune responses. The absorbance of TLA-treated splenocytes increased at days 3 and 6 PI but returned to normal at day 9 PI. The absorbance of con A or TLA-treated MLN cells was suppressed throughout the experimental period. 
Fig. 5Changes of CD4+, CD8+, NK, and macrophages among splenocytes (left) and mesenteric lymph node (MLN) cells (right) of mice intraduodenally infected with 106 T. gondii KI-1 tachyzoites. Cell phenotypes were examined by fluorescence-activated cell sorting (FACS). CD4+ T-cell proportion gradually decreased among the splenocytes and MLN cells at day 9 PI, whereas CD8+ T-cell proportion was not significantly lowered among the splenocytes and MLN cells at day 9 PI. There were no changes in NK cells or macrophages throughout the experimental period. 
Fig. 6Kinetics of IFN-γ and IL-10 produced by splenocytes of mice infected intraduodenally with T. gondii KI-1 tachyzoites. KI-1 infection induced an increase of IFN-γ levels at day 6 PI. The level of IL-10 did not change significantly during the infection stage. 
Table 1.Presence of KI-1 tachyzoites in mouse organs amd tissues as revealed by microscopy and PCR |
|
||||||||||||||||||||||||||||||||||||